Q: What do you mean, "can of worms about human activity and the second
law"? What has that got to do with simple old rusting iron?
A: Mainly that we usually don't want it -- except for iron ore (which is a mixture
of dirt or rock that has a lot of iron rust , i.e., iron oxide, in it). We really
like millions and millions of tons of that because it's worth millions and millions
of dollars! But before we start digging in an iron mine, let's look at how we
humans use the second law for our purposes. Whenever we run a truck or any kind
of engine, we're using the second law for our benefit:
[Note! From here on when I write "the second law", it is using those
words as a code phrase or shorthand for "what the second law describes",
namely: "some process in which energy disperses or spreads out"]
|
taking energy inside of substances that tend to spread out, but can't
because of Ea, |
|
(i.e, a high-energy mixture like oxygen (of the air) plus liquid
gasoline (or solid coal), |
|
giving it the necessary activation energy (a spark or a flame or heating
at high pressure), |
|
having the diffusing energy (in the form of hot expanding gases of CO2
and H2O) push a piston that turns |
|
crankshafts, gears and wheels (with the exhaust gases,
still fairly hot, but no longer |
| available
for any more piston-pushing in this engine going out the tailpipe). |
So in this example, it looks like
the second law is a good deal for us. It is, whether in engines or in our biochemistry
(where oxygen plus food is the concentrated energy source -- but with totally
different "sparkplugs" in our bodies to start our oxidation reactions
that are far more controlled and in very tiny quantities compared to the violent
explosions in a car engine!) Nature's second law predicts that the energy concentrated
inside a chemical mixture like oxygen with oil or coal (or food) tends to spread
out. It will do so, if that necessary little energy push to overcome
an activation energy barrier is applied to that kind of high-energy mixture
compared to the lower energy products of CO2 and H2O.
We make our whole technological world run by grabbing as
much as we can of the energy flow available from concentrated energy mixtures
like oxygen and fuels to run an infinite variety of machines, electrical generators
and vehicles. (Our bodies, as we have said, use second-law energy flow from
the oxidation of food for the synthesis of essential compounds and for all activity,
from biochemical to muscular to mental.) However, when we change energy from
one form to another, from energy in a fuel plus O2 to pushing a piston or even
water running down from Hoover Dam to the dynamos below, it is impossible for
us to get to use all of the energy in the concentrated energy source
for the jobs we want it to do. Some always is diverted as the unusable energy
due to faster moving molecules (i.e., "heat") to the environment.
(That's where our body gets heat to maintain our 98.6º F/37º C.)
Q: OK, I get it. Every time I start our car, I'll think of the energy the gasoline's
giving out by reacting with oxygen, making hot gases, pushing those pistons
and turning that crankshaft….
A: And every time you breathe, don't forget the oxygen going all over your
body and …well, let me do some more summarizing before talking about that
biochemistry angle a little more:
This minute all around the world there are tens
of thousands of people who are "using" (transforming to mechanical
work, losing some to waste heat spread out to the environment) the concentrated
energy of a mixture of oxygen with coal, oil or gas to dig up the iron ore with
giant scoops and transport it via trucks, trains, and ships from different mines
to steel mills. Then, more energy is used by more thousands of people to change
it into iron and finally to shiny steel...What a long parade of actions based
on using the second law to get what we want!
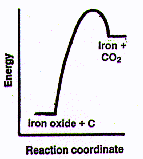
Every step from the original rusty dirt in the ground requires transformation
of concentrated energy (of oxygen plus coal, oil, gas) to do a lot of mechanical
work (along with that dispersing of less concentrated energy in the hot exhaust
gases of CO2 and water). Then bringing together thousands and thousands
of tons of ore, coal and limestone to one place, the steel mill, is another
enormous expenditure of concentrated energy in fuels (not counting the human
effort in muscle and brain). Next, a totally different variety of energy transformation
is done, changing the iron (oxide) ore to almost pure iron metal
that has a larger internal energy content in its bonds than does the
iron oxide. Wait a minute! Doesn't it seem against the second law to force a
dispersed-energy chemical like iron oxide to change into a concentrated-energy
chemical like nearly pure iron? Sure it is, but there's no problem. Just
as in running all those truck, train and ship engines, we can take energy flow
from a spontaneous process (here in this case, from two chemical processes):
The spontaneous reaction of carbon from coal with a little oxygen to
form CO whose molecules are moving very fast (i.e., are very hot), followed
by
the spontaneous reaction of CO with iron oxide to form fast moving
CO2 molecules plus pure iron and cause the nonspontaneous
change of iron oxide to iron. Of course, in doing that we will lose a large
flow of energy as waste heat. To give an idea of the size of it in iron making,
a ton of near-pure carbon (coke from coal) reacts with four tons of air at around
1000 C in a blast furnace to form a ton of pig iron from two tons of ore. The
energy price is six tons of hot flue gas that the process spews out,
some of which isn't available for more changing of iron oxide to iron. Pretty
big operation.
Did we beat the second law? No way. But by using the
second law (taking the energy from two spontaneous "downhill" reactions
and transferring much of it to force a nonspontaneous process to go "uphill"
energy-wise and make something), just as we take gasoline energy and change
some of its energy into mechanical energy (to make pistons, crankshafts, and
driveshafts turn the car wheels), we got what wanted: iron from which we can
produce steel, the structural material for a near-infinite number of useful
objects. Better than rusty dirt, right?
Q: Are you trying to make an iron man out of me?
A: No, no. Stick to the triathlon for that. The reason I went so long on that
kick of ore to iron is that it's a perfect summary of the tremendous variety
of what humans can do with the aid of the second law.
We gather objects and mixed-up raw materials from all over
the world. Just bringing stuff of all sorts from so many widely separated places
to one spot as in iron and steel making is certainly not a probable occurrence
in inanimate nature! It's a human act, especially when you consider the further
elaborate arrangements that we make with all varieties of matter, from lining
up botanicals in a National Arboretum in Washington, DC to joining metal and
many other kinds of materials into building a skyscraper in Chicago or a Getty
Center in LA: Those are big things.
Equally as spectacular are the human actions in smaller
things, bringing together the materials and fabricating a Boeing 747 or a jet
engine with so much power that a couple of them could move a Titanic. Gathering,
arranging, building, fabricating -- in all of these we use (what we can of)
the directional energy flow from spontaneous chemical reactions such as the
oxidation of petroleum and coal.
Let’s finish this recap of human use of the second-law
energy flow: Besides making concentrated-energy chemicals like pure metals --
iron, copper, chromium and silver -- from their diffused-energy ores (and innumerable
objects from them), we make thousands of other high energy substances for our
pleasure or our needs. Minor things like flavors for foods. Important pharmaceuticals
that save millions of lives. It may take dozens of reactions (milder than that
violent one for iron from iron oxide!) to change starting materials stepwise
to the final chemical product, but the overall process involves diverting energy
from spontaneous 'downhill' reactions to make the 'uphill', more concentrated-energy
substance that we want.
Of course, this is the kind of coupled process (i.e., a spontaneous + a non-spontaneous)
that nature uses – taking a tiny bit of sunlight energy and, with the
aid of extremely complex processes in organisms like plants, changing lower-energy
carbon dioxide and water and traces of minerals into thousands of higher-energy
substances. But don’t think that "natural" or "from natural
materials" has something to do with good or harmless! There are hundreds
of harmful or even poisonous chemicals in nature – from strychnine to
the extremely deadly compound in simple castor beans. (Also usually omitted
when someone extols the beneficial qualities of everything "natural"
is the fact that all terribly toxic viruses and bacteria are totally natural!)
—> Next
<— Back Home
—>> Last
