| Obstructions to the second law make life possible |
Roommate Q: I’d better ask questions here because my roommate said he was an expert in this and didn’t want to embarrass you. First, what IS an obstruction to the second law of thermodynamics?
A: What a considerate guy! All heart.
I admit that secondlaw.com/three.html told him all of the first part of what we’ll be talking about, but the last part here is new. I think you’ll enjoy it because you said a friend told you that "no complex molecule could be created without a mechanism". (I guess that implies that the second law says every substance always tends to go "downhill" in complexity as well as in energy. Also, nothing orderly could be formed spontaneously from the randomly moving molecules of chemical elements.)
We’ll see that viewpoint is totally out of synch with normal everyday thermodynamics – even though we certainly won’t say that proteins, carbohydrates, and DNA could have been easily formed spontaneously under primitive-earth conditions. Nothing like that!
Q: OK, I glanced at that page 3 of secondlaw site. Read about activation energies. How’s this? Most of the chemicals in our bodies and in things we like best – made of wood, metal, fabrics, paper, plastics, leather, rubber – are not thermodynamically stable in air (due to their reaction with the oxygen in air). That’s because the second law says that chemicals with greater energy inside them tend to form other substances with lesser energy and that difference in energy gets "spread out" via molecular motion (and light) to the outside world. So, because oxygen plus practically every chemical inside us and in the things we like have more internal energy than the oxides formed from them, we and all our stuff should burn up in one gigantic hot fireball.
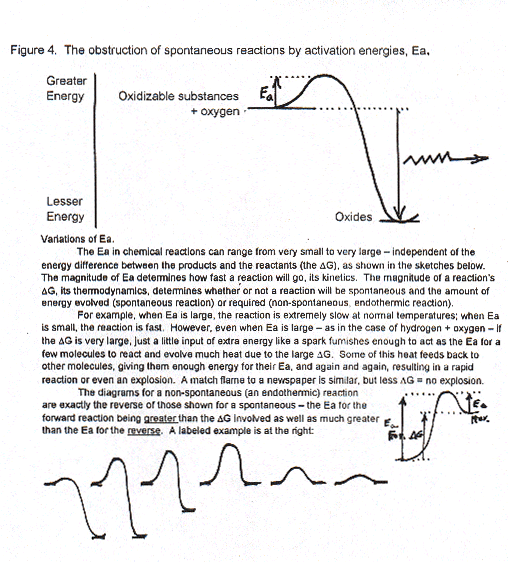
Fortunately, in ordinary chemical reactions the second law is obstructed and held back by what chemists call an activation energy, Ea. This is the amount of "extra" energy that has to be put into any set of chemicals to initiate their reaction. That Ea has to be added even though the chemicals may contain much greater energy than the products they would form. For example, we all know a tiny match flame on a big sheet of paper will start it to burn – or start a whole national forest burning that, chemically, means their cellulose and other substances are reacting with oxygen in the air. The little heat from the match is all the Ea needed. Good enough summary?
A: Terrific. I couldn’t have done better. You have now heard about the most sensational idea of chemistry that chemists just haven’t been able to get across to non-scientists: No life would be possible on this earth if it weren’t for the phenomenon we call activation energies, Ea.
Maybe it would help if I’d sketch a plot of the energies involved in what you’ve told me. Let’s put the greater energy-containing rubber/paper/steel plus oxygen at the left top, then the little energy bump representing that Ea needed to initiate the oxidation, and the drop down to the final lesser energy-containing carbon dioxide or other oxides. (The height of each point on the plot indicates its chemical potential energy.)
The quantity of energy that is being spread out by the substances reacting with oxygen – heat and light -- is equal to the downward arrow and its going out in all directions is represented by the zig-zag horizontal arrow.

That’s a typical plot for millions of possible oxidation reactions. But the second law can’t be obeyed (and "time’s arrow" seen to be fulfilled0) until that little bit of initial energy, Ea, is added to a system consisting of paper, or wood, or iron, etc. plus oxygen (from the air, except in lab experiments). THEN, the reactions proceed spontaneously, all by themselves, with a great deal of energy spreading out to the world outside the molecules.
Most of these oxidation reactions we try to prevent. We don’t want houses or forests to burn down, and certainly we ourselves don’t want to catch fire spontaneously! We won’t and they won’t if we somehow prevent the energy of activation, Ea, from being supplied to them or to us.
On the other hand, oxidation of gasoline, diesel fuel and jet engine fuel is tremendously desirable to make happen in a controlled fashion in our engines because engines can do thousands of jobs that we can’t accomplish just with our hands and feet. From the plot in Figure 4, we see why the spark plug in a car is important; it furnishes the activation energy needed to start the gasoline vapor reacting so rapidly with oxygen in the air that it explodes. (An explosion is just the rapid release of a lot of heat that expands gases.). The fast expansion of the residual air and other gases that have been greatly compressed by the explosion in an engine's cylinder pushes the piston down and that mechanical motion then turns wheels.
From this example we can understand how we are "using" the second law of thermodynamics for our human purposes when we burn fuels in engines – or oxidize food in our bodies. To do work that we want accomplished, we take advantage of the second law’s eternal prediction that energy tends to spread out from high-energy substances (like gasoline plus oxygen) or processes (e.g., water flowing down toward sea level) to lesser-energy compounds (like carbon dioxide and water) or situations (lower gravitational levels in physical events).
Remember, when we appear to be defeating the second law of thermodynamics by making greater-energy substances, like pure steel and aluminum, from their lesser-energy oxide ores, that is only half the story. The other half is like the diagram in Figure 3 -- we are actually using energy made available to us thanks to the second law and putting SOME of it into making new high-energy chemicals. I yell "some" because there’s a silent prediction of the second law that says we can’t ever achieve perfect transfer of thermal (or other) energy in any process. A little is inevitably converted into heat that is unavailable for our use at the temperature of the process.
Q: Yeh. That’s fine. I can see how we sorta use the second law for our own purposes in many ways, including just plain living in keeping our body going with food. But what’s that got to do with how complex compounds may have been formed billions of years ago on earth?
A: Lots. But first let me say what I’m not going to say! I’m not going to talk about the origin of life on earth or anywhere else. No one has scientific answers to that big question. Even the partial answers have huge gaps in them that are completely unbridgeable at present.
My concern is not with pre-biotic compounds or with primitive-earth pathways to proteins (good luck!) but with misinterpretations of the second law of thermodynamics. I’ve heard such crazy statements about the impossibility of such things as complicated molecules arising from simpler ones "without a mechanism", implying the necessity for human or divine intervention in each specific case. (Creationists certainly would agree that the second law is part of the original creation and surely they must include all the laws of chemistry.)
Unfortunately, many many people who have no knowledge of chemistry are making erroneous statements that involve the most basic principles of the subject to large audiences in speeches or on the Internet. Such misinformation gives a false basis for the hearers’ judgment. The correct foundation in chemistry is not complex and is fascinating in its many aspects
Atoms and molecules inherently attract one another. Among molecules, the attraction is usually weak. However, many kinds of atoms so strongly interact with one another that they "bond", i.e., form extremely powerful associations in very specific ways to yield molecules so stable that energy at temperatures of a thousand or two thousand degrees can’t tear them apart again. Molecules are not atoms randomly stuffed into tiny packages. When three or more atoms join to form a molecule, they are arranged in precise order, normally unchanging over time, and with a relatively fixed geometric relationship. Finally, many kinds of molecules can strike other kinds very violently and form totally new types of molecules – another mode of formation of new complex ordered structures due to the same innate nature of atoms to form strong bonds..
A simple example is the reaction of hydrogen gas (and perhaps aluminum paint on its cloth) with oxygen, tragically illustrated when the hydrogen-filled Hindenburg dirigible burned in 1937. Hydrogen has a great inherent tendency to bond strongly with oxygen and form water even a small energy of activation, in the form of a spark affecting only a relatively few molecules, causes the two substances to start to react, resulting in an enormous evolution of energy. This is exactly as the second law predicts: some of the chemical potential energy in hydrogen and oxygen molecules tends to be spread out when the less-energetic water molecules are formed. Yet, water is more complex than the simple elements and its atoms are arranged in an exact geometric pattern as all chemistry students know so well. (HOH is simply depicted, hydrogens in gray and oxygen, red, in the New York University link here. More complicated organic compounds with carbon in black can be found here.)
There are millions of possible syntheses like that of water being formed from the elements, i.e., there are millions of "intricate" compounds that have less energy in them than the elements of which they are composed. That sentence is a quiet bombshell. It takes a while for it to fizzzz before its significance explodes. It means that the second law energetically FAVORS -- yes, inexorably predicts – complex, geometrically ordered molecules can form from utterly simple atoms of elements. In such syntheses, the second law favors orderly, precisely arranged big units over the smaller, simpler parts. This is a fact, not an opinion. It negates popular statements such as "the second law says that all systems fundamentally tend toward disorder and randomness".
In the foregoing I am only pointing out the relationship of the second law to well-established and thoroughly-measured basic chemical energetics. I do NOT mean to imply that in the laboratory we can simply toss hydrogen, oxygen, carbon, nitrogen, and phosphorus in a reaction vessel and come out with a batch of DNA. No way! (The practical lab processes would be enormously more complex, impossible at present.) However, looking only at the energy relations, not the detailed steps of synthesis, we can see that virtually all of the 30,000 or so substances in our bodies would be energetically following the demands of the second law if they had been formed from the elements. Each of them contains less energy than the elements of which if is composed.
To summarize this important conclusion that is known by very few who are not chemists: Energetically, the second law of thermodynamics favors the formation of the majority of all known complex and ordered chemical compounds from the simpler elements. Thus, contrary to popular opinion, the second law does not dictate the decrease of ordered structure in its predictions, it only demands a "spreading out" of energy in all processes.
Also, to repeat the caution: The foregoing only describes energetic relationships involving the second law. It does not mean that most complex substances can be readily synthesized just by mixing elements and treating them in some way.
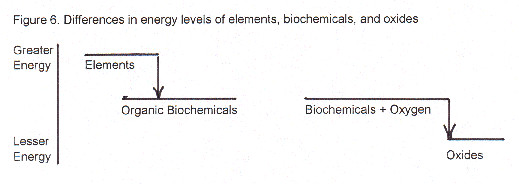
Q: Hey! Hold everything for a second: If most all the chemicals in our bodies are less energetic than the elements, our body chemicals are really really stable, right? Then if they are so much more stable than elements, like you just said, how is it that almost everything in our body could spontaneously burn in air (if it weren’t for activation energies keeping us from catching fire)?
A: Good question. "Stable" and "energetic" really are relative terms and have to be talked about in relation to the stability of other energetic substances/things/states. The trouble you point out is caused by not realizing that the initial and final states in those two situations are completely different.
Look at the diagram below with three energy levels, first (top) elements, second (middle) body chemicals, third (lowest) oxides. The initial state in your first example has the elements, higher energy molecules, on the top level and then "body chemicals" that have lesser energy content as the final state on the middle level. Then, in your seemingly contradictory example, your initial state is body biochemicals plus oxygen that have higher energy (biochems on the same middle level) than the final state of oxides formed when body chems burn to form carbon dioxide and water (plus oxides of the other elements in biochemicals).
Q: OK. You cleaned up that problem. How about getting on to the big question, "How could complex chemical compounds have been formed billions of years ago on the earth?" (I’ll bet you are going to sneak activation energies in there somehow!)
A: You're absolutely right! They're the protectors against destruction as predicted by the second law for chemicals that have a lot of energy inside themselves. Big daddy second law tends to make those chemicals go downhill so their energy can become spread out in the surroundings. Energies of activation continually tell big daddy to "Hold it! Don't let that energy go downhill yet."
To start, I must warn you again and then recap.
Warning: I’m not going to talk about the start of life on earth. Not at all. My aim is just to look at the energetics of compound formation in space and perhaps on the primitive earth.
Recap: You’ve already heard about the thermodynamic data that shows most complex substances contain less energy than the elements from which they are formed. Thus, the spontaneous formation of these complex compounds from their simpler elements is favored by the second law. This wipes out all the erroneous popular statements like "The second law says that complex compounds cannot spontaneously form from simple substances".
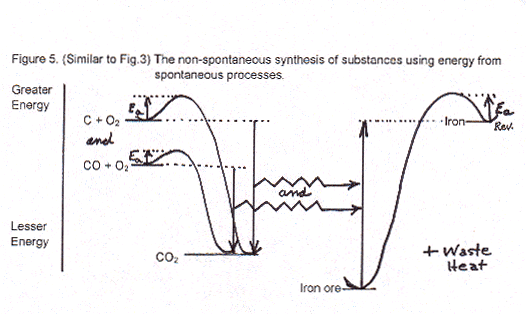
My goal now is to tell facts and propose mechanisms for the non-spontaneous formation of high-energy molecules that are found in space today and that might have been formed on primitive earth. Energetically, the second law absolutely, positively, certainly does not STOP complex, higher-energy compounds from being formed from lower-energy substances. Then, thanks to activation energies, if higher-energy compounds can be made, activation energies preventing those high-energy compounds from going back downhill makes it possible for them to continue to exist for a short or long, long time. The problem is, "How can they be formed?"
Your question about biochemicals being oxidized is a good start to recapping what we’ve seen about the synthesis of greater energy-containing compounds than their starting materials. Figure 3 showed a biochemical example (of ADP boosted up to ATP) and Figure 5 illustrated making high-energy iron from low-energy iron oxides (iron ore) by using carbon from coal and oxygen. Both figures were greatly simplified, of course. Both examples required some fancy procedures: enzymic catalysts in the biochem, very precise heating of the ore and partial combustion of the coal to CO in iron production.
Biochemical oxidation within us involves many many little steps in a huge number of different reaction types, all aided by complex enzymes. Overall, the result is evolution of energy. Much is not usable for any chemical process and thus is called waste heat, but still it serves the important function of keeping our bodies warm. The rest of the energy from oxidation is used to carry out necessary bodily processes including the synthesis of about 30,000 different compounds. Even if all these reactions to make compounds like proteins and starch and hormones and hemoglobin are not endothermic, they all require a bit of initial energy to surmount Ea barriers for their synthesis. Thus, energy transfer from spontaneous oxidation (catabolism) to non-spontaneous synthesis (anabolism) is essential. (Energy transfer of this sort between spontaneous and non-spontaneous chemical processes in biochemistry is called "coupling".)
Figure 7 is very very simplistic schematic of energetic coupling in biochem. A really good plot would have to be three-dimensional, not flat, and have to accommodate many more possibilities than 30,000 hills and valleys – like a 3-D cross-section of the whole Swiss Alps, including major rocks! It should also have "zig-zag energy arrows" all over the place to show coupling. Figure 7 is meant just to give a feeling for how endothermic reactions can take place because of energy transfer/coupling (plus the important aid of guides and catalysts that are enzymes).
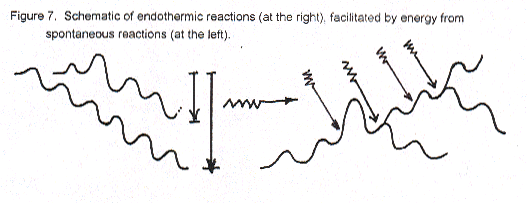
The foregoing figure again emphasizes that the second law’s directionality of energy flow (i.e., of energy tending to spread out) by forming lower energy substances is unconsciously used by us for the synthesis of our complex molecules.
Even though the particular substances in our body are enormously complex, and require complicated enzymes as catalysts, the basic energetic conclusion is that higher energy substances CAN be formed IF energy is supplied from outside them. In the primitive earth there was a lot of energy floating around, from volcanoes, hot vents in the ocean floor, hot pools, extremely powerful ultraviolet light (because there wasn’t any ozone layer to stop it then), and the impact of comets and big meteorites every million years or so.. These could have supplied activation energy to force unusual reactions.
How about the following sequence of reactions in Figure 8, where "hv" indicates an intense beam of high energy radiation hitting a molecule or group of atoms?
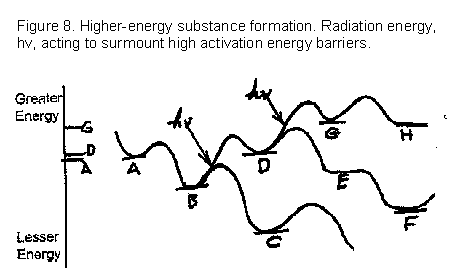
In Figure 8, substance B, in the presence of a little extra heat energy to provide activation energy, can then form C. However, if hit by powerful radiation, it can take another path to form the higher energy D. Subsequently, D spontaneously tends to form E and F but, if by chance irradiated, it forms G – a substance considerably higher than B or A in energy and, possibly, in complexity.
Just one new kind of energy plot for the formation of new molecules. Here's an...

Of course, there are millions of other possibilities for pathways. I am trying to illustrate only two major facets of the formation of new substances in the presence of intense energy sources and where other elements or compounds can react with the substance being examined.
1) The second law does NOT dictate that complex or greater-energy compounds cannot be formed. It only says that the incident energy forming them (i.e., energy from the surroundings) cannot totally be transferred to them because some must be lost as entropy in the process. It also predicts firmly that all substances tend to revert to their lesser-energy starting materials or to other such substances with lower energy content.
2) Any "greater-energy" substance exists for an appreciable time ONLY because of activation energies that obstruct change to lesser-energy compounds. An extremely important – and, so far as I have seen, previously unstated mechanical analogy is as follows:
Activation energies are the "pawls" that protect greater-energy containing substances, which are formed in a "ratchet-up" synthesis, from instant reversion to their lower-energy reactants. This is true in the laboratory or in a mixture in nature from a coincidental "energy ratchet-up" chemical process.
Q: Wow. Don’t you ever stop to take a breath? But the idea sounds simple. Give a chemical a kick of energy – or maybe be hit by another rapidly moving chemical’s molecules – and possibly it will form a new compound that has more energy in it. No way that’s against the second law because some of the energy boosting the chemical up and being caught by it is wasted in entropy, i.e., useless energy spreading out as lower temp heat. But the new substance can’t stay that way all filled with more energy unless there’s that little activation energy hill – or pawl -- that keeps it from sliding back down to where it came from, the way the second law says it ought to behave.
That could be possible in some hotshot lab. Does it really have anything to do with what might have happened on the earth a few billion years ago?
A: It sure looks that way. Do you know what compounds are in outer space today? In comets? In meteorites that have hit us recently? Those undoubtedly have been around since before the earth formed.
There aren’t too many types that are proved, not the hundreds and thousands that one might want to learn were pounding the daylights out of each other and forming a huge mess of other different and exciting pre-life chemicals raining on the earth. But dozens of such chemicals are known to exist in outer space, while here on earth, hundreds more have been formed in research labs by irradiation of substances that are probably present in comets. What I am saying is that the squiggly sketches I drew a while back are not only possible energetically, but paths remotely like them probably occurred and are still occurring in space. In space, the incredibly powerful radiation from x-rays to far ultraviolet, with the huge masses of hydrogen and carbon (and much less of oxygen and nitrogen) should have been and are able to produce many types of compounds.
In comets, besides water in the form of ice crystals, spectroscopy shows signals for kinds of higher-energy cyanides or isocyanides, -CN or –NC. In intergalactic space, besides enormous quantities of hydrogen and large amounts of carbon (and actually some microdiamonds), there are probably simple hydrocarbons (alkanes) and even long chain hydrocarbons according to spectroscopic results. The most certain, and perhaps most surprising, primitive compounds are PAHs, polycyclic aromatic hydrocarbons. Not only have they been detected throughout intergalactic space spectroscopically but they have actually been found in meteorites that have fallen on the earth.
Why aren’t there more different kinds in meteorites? Why can’t we see spectroscopic evidence for a thousand compounds? Probably for the same reason that any compounds are formed at all! The intensely powerful radiation in space that makes high energy compounds also can easily overcome the little activation energy barriers that protect them from returning to the elements from which they were formed, unless somehow they are shielded from such radiation after formation. PAHs, containing only carbon and hydrogen and having much greater energy in each molecule than C and H, tend to go back to their original lower energy element C and H if struck by the right quanta of energy. It’s only accidental that some molecules shortly after being made are hidden from that radiation by being behind a particle of dust or behind a bunch of carbon atoms that protect the new molecules from destruction. Or maybe the radiation was a transient sort, decreasing and then increasing due to the fast rotation of an unsymmetrical star.
Q: Is that all?
A: Is that all?? Isn’t that enough?! You see now that the second law of thermodynamics is a powerful tool for predicting what tends to happen in an incredible number of different kinds of events. In addition you now know the many ways it can be hindered by activation energies.
Both are keys to life, to us as living organisms. Without the directional energy flow predicted by the second law – from more intense, concentrated, or having greater internal content to diffused, spread out, or lesser internal content, we wouldn’t have the possibility of obtaining energy from food molecules, storing it in our ATP or similar substances, and using it for our chosen purposes. Without activation energies (or, technically, without the molecular mechanisms responsible for the phenomena), NO chemical substance could be stable even for microseconds.
You could go even further back -- to the formation of matter from energy after the big bang -- to be amazed at the generality of the second law and the importance of activation energies in the beginnings of matter. That's the domain of cosmologists and astrophysicists, but nevertheless that also is (or was) a domain ruled by the second law. The original incredibly concentrated energy of the universe did not stay in a small region. It tended to diffuse in space and to form lower-energy matter that further decreased the concentration of energy. That's really following the predictions of the second law big time! Also, comparable in effect to activation energies protecting chemical compounds, were what physicists call potential energy wells (PEWs) that protected fundamental particles like protons and neutrons from immediate destruction by the enormous energies present everywhere after the big bang.
Then, after protons and neutrons and other fundamental units were forced together by fusion to form elements (protected from reversion to the original particles by PEWs), we are on more familiar ground for the chemist. Fortunately, there were "mistakes" where hydrogen became compressed and resulted in suns where the fusion process didn't stop but continued to give out energy (that then followed the second law). Elsewhere, the enormous energies of the early universe (far less than shortly after the initial big bang) could cause elements to unite and form the lesser-energy solid matter that is still present in the earth and planets (e.g., the ubiquitous silicates of rocks) -- higher energy elements forming lower energy substances, just according to the second law.
Subsequently as well, the elements and simple compounds could be transformed into those that were more complex under the purview of the second law and the protection of activation energies.
That’s a remarkably powerful insight about how the world worked……and works.
Links
As mentioned at the beginning of this section, an extended development of the idea of activation energy is in secondlaw.com/three.html.
Basic thermochemical data is available from the National Institute of Standards and Technology: http://webbook.nist.gov/chemistry/ (Because for many substances the NIST tables cite only ΔH and S rather than ΔG, the reader should be capable of working with the Gibbs equation.) Note the printed references to thermochem datat given below.
Excellent information about molecules in space is available online from NASA Ames Research Center at http://www.astrochem.org.
References
The most complete, quickly "scannable" tables of thermochemical data are in Professor Norman C. Craig’s Entropy Analysis (John Wiley, New York) 1992, pp. 172 – 200. Other less extensive – and thus less valuable – tables are in most physical chemistry texts.
Of course, the CRC Handbook of Chemistry and Physics Section 5 of thermochemical data is far larger than any text could be.
As stated in a previous section, Entropy Analysis, is the best short technical introduction to the laws of thermodynamics and the correct utilization of entropy in print. It is accessible to a diligent first-year college student, and especially valuable to a student beginning physical chemistry thermo.
Blum wrote the first article I know that introduced energy diagrams emphasizing the importance of activation energies as critical in the production of more energetic substances from lesser: See page 479 in H. F. Blum, American Scientist, 1961 (51), 474 – 501. (Online at blum1961.pdf) (I would appreciate additions to this reference to create a useful chain.)
Biochemistry Professor Robert Shapiro’s Origins (Summit, New York 1986) is an excellent rational summary of the scientific problems in establishing the origin of life on earth. There is much scientific myth-making about a few not-too-successful experiments. Creationists are wrong about almost everything involving chemistry but they are right about a big gap between the Miller-Urey experiments and RNA-based biochem.
| Home | Back | Next page – "The second law of thermodynamics and evolution" |