Q: Didn’t you tell us all about that tendency stuff in secondlaw.com?
A: Sure did. This page isn’t for you. You read that Web site, so you’re an expert and you can take a break. This is for your English lit roommate who just walked in -- because there’s so much balderdash in literature and the arts on entropy and the second law as being all about "disorder" and mixed-up human situations. It’s not. Hi roommate.
Roommate: OK, now that he’s gone, what IS the big deal about "tendency" and the second law? I’ve almost had it up to here with his talking about the second law, even though it seems important somehow. Isn’t it a good prediction? Aren’t things everywhere falling apart by themselves? Doesn’t wood rot, and steel rust and buildings crumble? Everything goes toward chaos? Entropy wins?
A: Sigh. Could he really have said that, after all our talking? But no matter what he said, that’s the way you heard him so let’s take it from there.
First, don't ever use that bad seven-letter word entropy in speaking or writing again if you want to be intellectually honest in your specialty of English literature (smile) . What entropy really means was described in the previous page on this site. It is ONLY applicable to events involving molecules and energy, not personal relationships or drunken parties in Chicago or society going to hell in a handbasket. Most writers foolishly embarrass themselves by tossing around the word "entropy". It's stupid to use as a metaphor and think you're really talking about scientific entropy. It's just as bad as if they would write that Einstein's theory of relativity applies to their rotten relatives. Ridiculous. Freud said, "Sometimes a cigar is only a cigar." A chemist would paraphrase Freud with, "Messy rooms or mixed-up human situations or random musical notes are messy rooms, human situations, or random notes. They are NOT examples of entropy!"
Now back to our important talking about the second law of thermo and everything falling apart: Give me a couple of insights about what you see in the world around you. Did you ever plant a seed? Did it "change into chaos"? Nothing green? No complicated flower or vegetable? Ever see a baby grow up? Ever see bulldozers build a slick freeway? Heard about complex chemicals in outer space? Those are all examples of chaos?
Interruption: Yes, yes – all that stuff. Sure, kids are born and get bigger and plenty of different kinds of trees and plants are around. But all people die and everything growing does too and it turns into dust, like the Bible or someplace says. (And neat new freeways get beat up with trucks or cracked by freezing.) Isn’t that going toward chaos?
A: Hmm. You’re certainly right that living things don’t exist forever. (You unknowingly illustrate my point by saying that energetic trucks and powerful earth movements are needed to wreck freeways! They don’t fall apart by themselves.) You know what? The different viewpoints that you and I have are like the oldest cliché there is – but we’re thinking about it dynamically. It’s the old story of the half-full glass of water on a table that an optimist and a pessimist are looking at. The optimist says it’s half-full and the pessimist says it is half-empty.
Our situation is different. We’re sitting in a dark room with the water in the glass spotlit in front of us on a table with a black tablecloth. Behind the table is a black drape that hides some apparatus like a water pump or reservoir and there are tubes we can’t see going into the glass and coming out of it so the level can be lowered or raised by a mechanism behind the curtain. Because the level of the water can be changed as we think and talk about it, this is a dynamic situation. Changeable.
Your statement about the second law is like saying that the level of the water in the half-empty glass will change in only one way, to go down toward completely empty because of the mechanism behind the curtain, i.e., things won’t stay as they are; they tend to deteriorate as predicted by the second law of thermodynamics. Maybe I’ll surprise you, and summarize this whole page, by half agreeing with you. But it’s only because you use that word "TEND"!
I see the glass of water as half full in that the earth is crowded with examples of complicated high-energy things that last – people for 70 – 80 years, trees for centuries, races of people and some human constructions for thousands, and complex compounds here and in outer space for billions. Keeping on with that water metaphor, I see the total water level increasing on the earth even though individual "molecules" may "evaporate". (Sure, all living organisms die sometime. Yes, once in a while earthquakes bust buildings.) Yet, all babies don’t die as babies and not all the buildings are knocked down in a city with an earthquake.
Doesn’t that mean that there is a mechanism which prevents the bad predictions of the second law from coming true instantly everywhere? It must get postponed in a whole lot of ways or we humans wouldn’t be here and nothing that we make would last a minute. The second law may be a law and may have stern commands or predictions but they certainly can be obstructed or thwarted or hindered for a lifetime or a millennia and longer. (How that occurs is what secondlaw.com talked about and what the next page will explain briefly.)
R: So? What’s your point?
A: That we should be aware that the second law isn’t dominant immediately in all situations. It is short-sighted to concentrate on the fact that energy of the right type and large enough quantity can break or destroy all physical objects. Everything is NOT quickly falling into decay or collapsing all around us because there is some dread defect that is inherent in all matter. There is no "law of disorder" written into the nature of things in the world. It takes energy to cause wood to rot, oxygen and moisture before steel will rust, it takes earthquakes or tornadoes or gravity to make houses collapse or slide downhill. The second law tells what energy tends to do sometime in the future -- and, though I admit that in many cases (like a hurricane!) the future can be only seconds, I think you have to admit that in many cases the future can be billions of years.
Fortunately, because it is only a tendency and not an immediately executed edict, it can often be easily postponed or used to our advantage for a brief time or for a mighty long time.
R: The second law can be used to our advantage? Come on. My roommate wrote something on the wall that I couldn’t help seeing a hundred times: "The second law says that energy tends to flow from where it is localized or concentrated to where it is spread out or diffused – and it’s in that energy flow that things get messed up" That fits hurricanes and car crashes and explosives, but how can that be used to our advantage?
A: You’re using it now to your advantage, in thinking and breathing, and talking and living. I’m glad your roommate got one thing right. From his great writing, you can see that the second law demands that any spontaneous energy flow is directional: it has to spread out, only to go "downhill", never spontaneously in the opposite direction, "uphill".
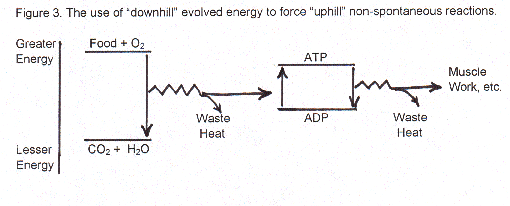
You eat concentrated energy in the form of molecules of food . You spontaneously oxidize them, a "downhill process", and while their energy is spreading out inside you as per the second law, you use some of that energy to non-spontaneously push some of your molecules "uphill" energy-wise to make one of your energy reservoir molecules, ATP, (plus thousands of other substances).
At the same time, according to the second law, you have to lose some of that energy in the food molecules being oxidized as entropy and that’s why your body is warm. (Every time you make an "uphill" compound by using energy from a "downhill" process, you have to spread out a little "downhill" as entropy.) ATP molecules are the major energy source in your body, used as the fuel for many processes of living. When you talk or breathe, you take ATP and change it to ADP to release energy – a "downhill" directional process dictated by the second law – and use the energy released in that spontaneous process to work muscles for breathing and thinking and speaking (while losing some as waste heat, that we measure as entropy increase).

R: So the second law is not all bad? The direction that it tells energy to go helps us as well as hurts us – we really can take advantage of it?
A: Absolutely. Check "The second law is the biggest good and the biggest bad on earth" on secondlaw.com/five.html The second law is a tendency. It’s a threat to us continually. But it can be forestalled and hindered and obstructed. And if there weren't any directionality to energy that the second law describes, energy could be totally random in its direction. If that were the case, neither we nor any stable universe could exist.
In addition to telling a little about the obstructions to the second law that have allowed life to continue, the next page also gives a way in which high energy compounds are probably formed in space and could have formed in primitive earth "against" the second law.
Next page –"Obstructions to the second law make life possible " |