Q: I get it. It's "adequate" energy flow that hits things and shoves
them around to look disorderly -- things don't do anything like that by themselves.
But wait a minute In some class or other I heard the prof say "everything
is going downhill to maximum entropy, to chaos and disorder"
A: Some profs say weird things! That's a century-old misstatement that may
sound profound but actually is just plain ignorant. It's like he took your
class to a horse show but let you look only at the rear ends of the horses.
Let's look at the rest of the horse instead.
You know all about the second law, how it says that
spontaneous reactions "go downhill" -- meaning that they all spread
out energy, and so entropy is always increasing. (Because entropy measures how
much energy disperses at a specific temperature, T.) So in all such processes, sometimes
the energy spreading out is "adequate" to push things around a little
or a huge amount. Maybe we think that's undesirable disorder, or maybe it's
obviously disastrous destruction.
Stop there a minute! Tornadoes and hurricanes in the
US are often horrendous -- their powerful energy flow certainly causes
chaotic wreckage, especially in towns or cities. What's the source of that energy
flow? It's energy from the sun hitting different kinds of the earth's surface,
including oceans (where water is evaporated, rises to high altitudes and forms
clouds). Because of the earth's varied surfaces, that solar energy heats the
air near them differently, so the rising air moves at different rates. Energy
dispersing; entropy increasing. We feel that as slight breezes or even strong
winds blowing here and there. Is that bad? (That's somewhat "disorderly"
but not really "chaos" or there wouldn't be any meteorologists and
TV weather people!) Occasionally in the US, powerful colder winds from northern
regions collide with equally fast-moving warmer moist air from the south and
for a few hours tornadoes are formed here and there. Not good at all.
But compare the number of those undesirable
events with the uncountable days of normal flow of moist air with clouds that
bring rain to different regions of the US! Those many many days of rain all
over the continent (and all continents) -- caused by "adequate" solar
energy pushing millions of tons of water from the oceans up in the air -- is
absolutely essential for plant life. Are growing plants -- from wheat and corn
to flowers and trees -- undesirable "chaos and disorder"? And that's
just one facet of the good that the sun does -- sending to the earth
only a billionth of the huge quantities of its energy it disperses per second
-- even though, thereby, causing an enormous increase in entropy in the
sun as well as a tiny fraction of that entropy increase on the earth. However,
we must always be aware that the most sensational downhill spreading out of
solar energy (entropy increase) is the small fraction that is coupled with the
uphill process of photosynthesis. Our whole lives -- almost all life totally
depends on that capture of solar energy as it disperses.
[[There is more readable information about energy coupling in biochem
toward the end of 2ndlaw.com/obstructions.html and by clicking on "Photosynthesis"
near the end
of the entropysimple.com introductory page.]]
And that "uphill"aspect of the second law -- coupled with activation
energies that protect systems from going back "downhill" energetically
-- is what most professors who are not in science simply don't realize.
Q: Hey! I get it -- that's the end of the horse that they don't know about because
they don't know chemistry: "The second law is our "greatest good"!
You said all that back on page five.
A: Sure did. Glad you realize that big idea from science.
Think what a wonderfully different place the
earth is today, compared to what it was a billion years ago when it was truly
chaotic and disorderly. "Everything going to hell in a handbasket",
some profs say? Activation energies hold the handles of that "handbasket"
so everything doesn't go to hell in so many instances! Today, full of amazing
"uphill" plant life and "uphill" life of all sorts plus
all the beneficial human-created order in small and large things, the earth
is a remarkably better place than as the hot hunk of matter it began.
A major reason that our present physical world is
deteriorating, of course, is human over-reproduction and overconsumption that
results in the degradation of our environment. But those problems aren't due
to some inexorable mysterious force or the Silent Sinister Subtle Scheme of
entropy! It is we who are causing them.
It is true that the sun is decreasing in mass as thousands of tons per second
are changed into truly huge amounts of energy. The sun is "going downhill"
but it will take more than four billion years to get to that hill's bottom.
That's a pretty long time off. I'm a lot more worried about the next fifty or
hundred, aren't you?
Q: Sure. The next two. I have a feel for entropy. But why did you go into
such a big song and dance with that jagged diagram a while ago just for busting
a surfboard, skateboard, snowboard or ski??
A: Add "or for a car fender, jet engine blade, freeway bridge, leg bone,
spine, or skull"!! Doesn't that mean you?! That diagram covers breaking
an enormous span of solid things that really are important to us. So, it is
a pretty big deal for our understanding bad things that can happen in our lives.
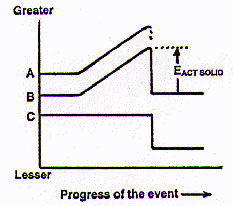
See that arrow, the EACT SOLID on the diagram? That represents a
function which is equivalent to the Ea that starts chemical changes,
chemical reactions like burning cellulose and rusting iron. The EACT SOLID is
the activation energy that has to be applied to any solid object to start
a physical change., its fracture. So the powerful new idea we have here is
that both for chemical changes like fires AND for physical changes like breaking
things, there is a minimum amount of energy, an activation energy, that is
required to initiate the process.
With that new idea we can broaden our previous statement
about activation energies in chemical reactions being obstacles or hindrances
to chemical change. (As we've said, all of the organic compounds in our bodies
could immediately catch fire in the oxygen of air if there weren't such obstructions
to the second law as Ea.) Energies of activation in solids, EACT
SOLID, similarly prevent solid objects from being broken readily. ("Wear"
in solids is breaking off clumps of atoms so the EACT SOLID is very
very important in thinking about tires or gears or bearings or anything wearing
out. When things don't wear out very fast, it means that inherently
they have relatively high activation energies against fracture occurring.)
(Actually of course, as we talked about before, it is the resistance
of chemical bonds to being broken that is reflected in the energies of activation.
Thus,Eaand EACT SOLID are indicative or derivative functions
-- i.e., they are due to a number of factors involving chemical bonds but
they can be used as shorthand or symbols for resistance to change.)
Q: So what?
A: So everything! Here comes the big payoff: How knowing about the second
law and activation energies can make a big difference in our understanding
bad things that happen to us.
—> Next
<— Back
Home
—>> Last
