

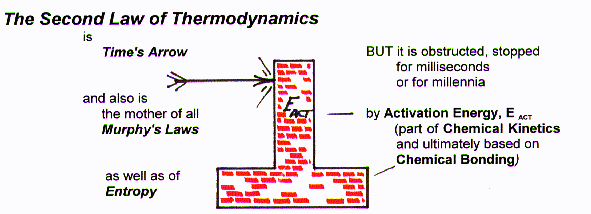
Time's Arrow Murphy's Law Activation Energy Chemical Kinetics Chemical Bonds
| This site shows that some ancient questions about "things going wrong" in our lives |
| have surprisingly simple answers in modern basic chemistry |
| (even things happening to us which cause that painful cry of "Why me?") |
| Still more important to one's philosophy about life, these chemical ideas can startle |
| us into seeing how fortunate we all are: that things don't go wrong more often! |
| We'll talk mainly about down-to-earth chemical reactions - like burning and rusting - |
| and the behavior of things - common solid objects of wood, metal, and bone, |
| not about complex computer chips or programs going wrong |
| (nor about personal relations that fall apart. Even chemistry has limits.) |
| Simple chemical reactions often are involved in annoying or deadly happenings to us: |
| being in a fire in Malibu, flying the X-1 (that broke the sound barrier – but had a |
| dangerous corroded battery cable), or things just “go wrong”: our tire blows out |
| a friend riding a horse and is thrown – ending up with a broken neck. |
Why? Why do we get harassed or even have our lives ruined by events like these?
Life is hard. But it's harder if you don't know how the material world works!
Here are some of the powerful keys to understanding from chemistry:
Chemistry students: If you're in a time bind or an exam is coming up,
go here
for a shorter approach to understanding
the second law and entropy.
| Frank L. Lambert, Professor
Emeritus Occidental College, Los Angeles, CA 90041 Academic and professional biography entropy.lambert@gmail.com |
January 2011 |