Q: You're whipsawing me. A while back you said the second law
was the mother of all Murphy's Laws. Now you show me that the second law is
a good buddy because we can use it for energy to do what we want. That's double-talk
isn't it? What's the story?
A: Come off it. You're not naive. Life is full of stuff that can be either good
or bad. But get ready for a shock now: [Remember what I said about the words
"second law" -- that they are often code words for what the second
law describes, i.e. that energy spreads out, if it can, from being
localized or concentrated to becoming dispersed.]
The second law is the Greatest Good and the Biggest Bad to us.
The GOOD: Because of the second law about the direction of energy flow, life is possible.
The BAD: Because of the second law -- the direction of energy flow -- life is always threatened.
Most of the compounds in the feedback systems are also synthesized internally by thermodynamically nonspontaneous reactions, effected by utilizing energy ultimately transferred from the metabolism (slow oxidation) of food.When these feedback subsystems fail -- due to inadequate energy inflow,
How's that for starters? You can't get any better for good -- that living
is possible due to the second law. And you can't get much worse for bad -- that
death is always possible too, due to the second law.
Q: But what happened to Murphy's Law? That isn't about death, just about less
bad things that hit us.
A: You're right. Murph doesn't get that serious very often, but there are at
least five thousand illnesses, diseases, "things that can go wrong"
with our bodies that may not kill us. That's 5K of
Murphs. These are biochemical problems that humans suffer from. But how many
do most people have? Did you ever see a PDR Medical Dictionary or an AMA Home
Medical Encyclopedia? They'll make you very thankful for activation energies
and feedback systems that keep your bod working as well as it does (and long
as it will) to counter the second law, using food and oxygen intake as your
energy source.
However, let's look at the other annoyances (and disasters)
that the mother of all Murphys is responsible for when things that are around us have energy concentrated inside
them. That's always potential big trouble. All that has to happen, somehow,
sometime, is for a little energy push -- a spark, a flame, an impact -- to get
up over that activation energy hill. (Remember the energy diagram for cellulose,
i.e., wood and paper? It applies to anything flammable and literally millions
of other oxidation reactions, e.g., iron or any metal rusting or corroding because
rusting is oxidation.)
First, problems caused by the thing or material having concentrated energy inherent
in its chemicals:.
Trees catching fire
a house struck by lightning
a curtain too near a candle...
the forgotten cigarette left on a sofa
Mrs. O'Leary's cow kicking over a lantern in straw and burning half of Chicago
the spark from a bulldozer that started a grass fire and then a forest fire
These are all cases of exceeding
an activation energy, resulting in a spontaneous reaction.
And, of course, there are many less (or
equally) dramatic examples in the oxidation of metals
Rust on a tool, disfiguring or damaging it
rust in a machine, hindering operation
copper oxide in an electrical socket, causing overheating and then a fire
battery cable corrosion in Chuck Yeager's X-1 that almost killed him.
Second, annoyances (or worse) due to concentrated energy in the object being present or
flowing by it, but not inherent or part of its nature:
Tires that blow out
hydraulic brake
systems that leak suddenly under pressure
audio speakers that are fed high wattage signals 230 volts
into a 115 V house circuit
winds in the air.....from gales to hurricanes, from windstorms to tornadoes.
A car going 80 around a 30 mph curve, a 747
hitting a mountain, an Indy car into the wall.
Q: Yeh. Yeh. I get the point. Or points. Know too much about car crashes. New
to me, before we began to talk, was to hear that burnable stuff, like wood and
paper and cloth in my room
(along with the oxygen of the air) is basically a bunch of concentrated
energy chemicals. But I don't have sparks or candles around to give them an activation
energy kick and cause a fire. Breaking things is more of a problem to me.
Is there energy locked inside a skateboard or a ski that wrecks it (and me)
because it tends to diffuse or spread out?
A: Good comment and good question. It's great that you now understand why
certain things can react with oxygen and why a spark or low flame sets
off a spontaneous reaction. You also know now that all of these kinds of problems
from fires to plane and car crashes to lightning to tornadoes and fires are
related by the second law of thermodynamics: concentrated energy tends to spread
out. (A fast moving car is a "reely reely big" bundle of concentrated
kinetic energy.)
Your question about breakage is just as important because that kind of incident or accident happens to us more often than "Murphy problems" of fire that is due to energy concentrated inside the substance of the object and oxygen.
Breaking things involves concentrated energy that is initially outside the thing that gets broken. It's the second law working in the environment of the object -- energy flowing around or through it for some reason or other and hitting it with enough energy and of the right kind to tear it apart. (Right kind? Right amount? Heat won't make a concrete bridge shatter into fragments in thirty seconds, but a strong earthquake will.) Chemists never talk about breaking things because they don't consider that to be a chemical process. The chemical nature of a ski that gets broken, for example, isn't changed. It's just two skis so far as the chemicals in it are concerned. (Try to tell that to the skier!) Technically, the chemical composition of the two pieces of ski is almost the same so chemists call a fracture a physical process.
However, in a micro sense it is a chemical process because
in any break chemical bonds are ruptured all along the line of the break as
well as complexly broken and reformed near that break line. It's just that the
number of bonds altered is extremely small compared to all the others in the
ski that are not affected and therefore a chemist would never be able to measure
any composition change. Also, where and when the break will occur depends on
so many factors that aren't what chemists call fundamental, such as: how the
object was made, its shape, its ratio of surface area to volume, the strains
and defects present in it, whether it is brittle or ductile and even the rate
of application of energy to it. 
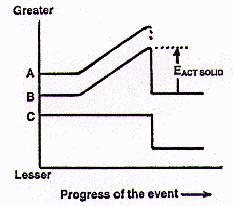
But we can plot the effect of a load (mechanical force) being applied to a solid object until it breaks. (Let's choose something that is especially valuable or useful.) In the diagram at the right, the line A represents the external load on it (the "mechanical force", that is, the effect of energy striking the object); B shows the internal energy of the object; and C is a rough estimate of the "human desirability" of the object (what it is worth). All the lines (A, B, and C) are initially horizontal to indicate their respective reference states before the application of any external force or load. As the load on a particular spot on the object is steadily increased, the internal energy of the object (line B) increases regularly with the greater and greater load (line A) bearing on it. If the external load acting on the solid is increased until fracture occurs, Line B immediately falls to the starting internal energy value (except for transient heat and the quickly dispersed kinetic energy in any flying fragments).
The difference between the high point of Line B and its original (and final) energy level is labeled in the diagram above as EACT SOLID . This is partly like an Ea , an energy of activation in chemistry. An Ea is the amount of energy required to start substances reacting. Then they continue to react spontaneously because of the considerably greater amount of energy evolved during the reaction. In contrast, an EACT SOLID is both the energy required to start a fracture and virtually the same amount of kinetic energy given out by the two separated pieces of solid.
Line C drops radically after the break, a rough indication of the far lesser value to us of the two broken pieces as compared to the original object. (Market economics, i.e., the value/price of the object before and after the break, best describes what line C represents.)
That diagram above is for a single break of a solid object. In a hurricane, wind energy is successively applied to the two fragments of the first break so that houses become scattered parts; boards often are torn into splinters. In the terrible 1995 Kobe earthquake, even concrete structures were torn apart and many portions of them reduced to rubble. At each successive step, the qualitative diagram applies -- additional load is supplied to fracture parts of the original and then those parts are again broken.
Oops. Splintered boards. Rubble. I'm afraid I have to talk about it.