Q: The second law and Murphy's Law -- they're related?
A: Yes, but I shouldn't have mentioned it. First, we need to know more about
bad things that can happen to us because of the behavior of chemicals in the
objects around us. For a grim example, wooden houses burn down with disastrous
financial loss even when people aren't killed. What's going on here in terms
of energy flow?
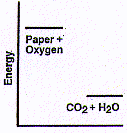
Wood and paper are both primarily cellulose. Paper is easier
to experiment with so let's think about its burning in air. When paper catches
fire and burns, there's a lot of energy given out as heat and some as yellow
light. It's well known now that the products of the combustion of cellulose
with the oxygen of the air are carbon dioxide and water. (The slight amount
of black ash is due to the clay that was on the paper adsorbing a small amount
of carbon.) Once started, the burning is spontaneous --i.e., the process goes
on by itself without any further help after a match starts it -- and also the
burning is really fast. Now, if all that energy is flowing out in this reaction
of paper with oxygen, the paper and oxygen must have had a lot more energy inside
them before the reaction than do the carbon dioxide and water after
the reaction -- as shown in the diagram above.
What's happening here is a beautiful illustration of the
predictions of the second law. Systems (groups) of chemicals -- or objects made
from them (like sheets of paper or houses) -- tend to react if they
have more energy bound inside their molecules than do the reaction products
that they can form. [[Note for Advanced students: "Energy change
here means 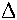 G,
not just
G,
not just 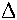 H".]]
Thus, when systems react spontaneously, their reactant molecules are spreading
out their internal energy in two ways: 1) part to the bonds in each molecule
of the products because each of those has less energy concentrated in it than
was in the molecules of the starting materials, and 2) a part to making all
those product molecules move much faster (i.e., they have more kinetic energy
than the original cellulose and oxygen). These faster molecules show a high
temperature on a thermometer. We say they are hot, not because heat is a "something"
but because heat is the process of energy transfer from one kind of matter to
another -- from fast molecules of gas to the thermometer bulb or to one's hand
if you get it near the flame.
H".]]
Thus, when systems react spontaneously, their reactant molecules are spreading
out their internal energy in two ways: 1) part to the bonds in each molecule
of the products because each of those has less energy concentrated in it than
was in the molecules of the starting materials, and 2) a part to making all
those product molecules move much faster (i.e., they have more kinetic energy
than the original cellulose and oxygen). These faster molecules show a high
temperature on a thermometer. We say they are hot, not because heat is a "something"
but because heat is the process of energy transfer from one kind of matter to
another -- from fast molecules of gas to the thermometer bulb or to one's hand
if you get it near the flame.
Q: Wait a minute! Wait a minute!! You had to put a match to that paper to start
it burning! What's spontaneous or second law about that??
A: Wait a minute yourself. Have you already forgotten that essential word "tends"
in the second law?
All the paper and wood and things made from them in the entire
world tend right now to react with the oxygen in the air and form one
gigantic fireball. Why don't they? Well, why don't all the mountains on earth
spread out the potential energy in their high stone cliffs this second and collapse
into spread out much-lower mounds of sandy particles? It's the strength of the
chemical bonds (between silicon, oxygen, potassium, aluminum and other atoms
and ions) that holds stone together and acts as an obstacle to the second law's
immediate execution. The potential energy of high rocks/mountains is hindered
from spreading out instantly.
Just so, the strength of the chemical bonds
(between carbon, hydrogen and oxygen) in cellulose holds it together and obstructs
the instant spreading out of the energy inside the cellulose by reacting with
the oxygen of the air. This strength prevents oxygen from instantly breaking
into the cellulose molecules to form the even stronger bonds (of carbon dioxide
and water) and to release large amounts of energy. However, it takes just a
little extra push of energy from the hot match flame to begin to break a few
quadrillion bonds in the cellulose of paper or wood so that oxygen molecules
also striking those 'breaking bonds' can complete the breaks and make the first
'gazillion' CO2 and H2O molecules.
The initial energy push
(usually from heat), shown by the small energy "hill" in the diagram
below, is the activation energy, Ea, that is necessary to overcome the bond-strength
obstacle to the second law in most chemical reactions. Thus, this requirement
for input of an initial energy, the energy of activation, hinders both desirable
and undesirable reactions from occurring.
An important idea is "Activation energies protect
substances from change."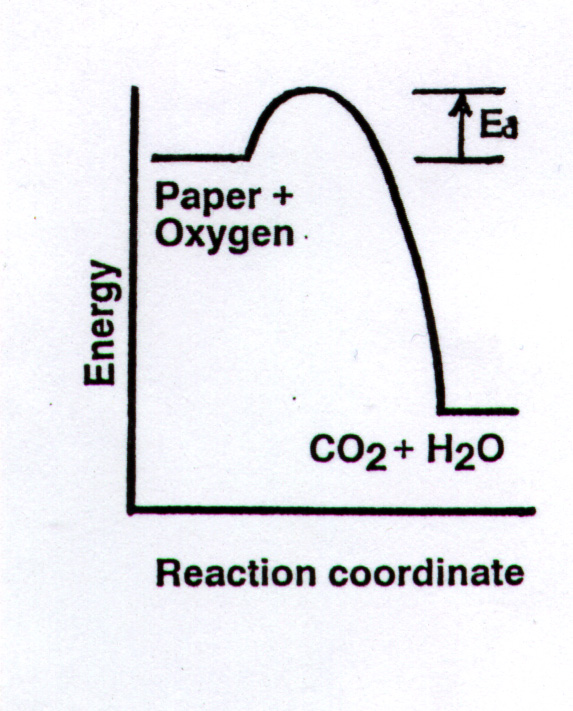
As these first "heated up" bonds are breaking, the oxygen from the
air begins to grab carbon and hydrogen atoms to form carbon dioxide and water
molecules. But the formation of new strong bonds in the CO2 and water gives
out a lot of energy -- enough to start to break many many more quadrillions
of bonds of cellulose (no bond being totally broken before oxygen has simultaneously
begun to form a new CO2 and water molecule from the developing fragments). These
new molecules of CO2 and water also absorb some of the energy from the new bonds
as they are formed and many move faster than twice the speed of
sound. We sense those fast moving molecules as hot gas and we call it "heat".
Q: Aha. I remember that in the Malibu fires a few years ago some houses started
to blaze from the inside because heat from the nearby burning trees and brush
ignited the cloth drapes inside the picture windows. Then there were others
with big windows that didn't catch fire because they had aluminum blinds which
were closed. That involved activation energy, right? Cotton cloth is cellulose,
isn't it?
A: Yes to both questions. First of all, the glass of the windows probably got extremely
hot, both from the heated air of the fire and the fire's infrared radiation. In addition,
as you suggest, the intense IR radiation went right through the windows and heated the
fabric drapes even more -- enough to exceed their activation energy that normally hinders
their oxidation in air. They began to burn and this gave out enough energy to ignite the
whole interior -- by exceeding the activation energy of oxidation of all the other
flammable materials in the house.
Just as does every idea that we've been talking about, the
concept of activation energies gives us tremendous power in understanding how
the world works, even in unusual events. 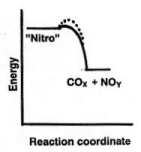 For
instance, you've heard about the dangers of nitroglycerin, a liquid that explodes
violently just from being shaken hard or jarred sharply. Do you think that its
energy diagram would look like the one for cellulose above? Of course not. It
must have a very low activation energy, Ea. That leads to an extremely
fast formation of hot gaseous products, an explosion (despite the relatively
smaller difference in energy between "nitro" and the products).
Explosives form hot gases so rapidly because they all have oxygen atoms as part
of their molecules. Thus, those molecules don't need to wait until some molecules
of atmospheric oxygen happen to hit them -- the way most substances have to
do. Alfred Nobel was driven to invent a safer explosive when four workers and
his brother were killed in the family nitroglycerin plant. He made what he called
"dynamite" when he mixed oily nitroglycerin with some powdery silica
material to form a seemingly dry solid that could be pressed into stick shape.
They didn't detonate just from being hit or dropped. Obviously, therefore, an
energy diagram for dynamite must look like the dotted line, a considerably higher
Ea indicating that more energy must be put in, e.g., by a blasting cap, to initiate
the spontaneous decomposition of the nitroglycerin. (TNT, used in armor piercing
shells, is about six times more resistant to shock than nitroglycerin. Thus,
you can guess at TNT's activation energy for reaction.) Dynamite has been
mainly replaced by other explosives for excavation, etc., today.
For
instance, you've heard about the dangers of nitroglycerin, a liquid that explodes
violently just from being shaken hard or jarred sharply. Do you think that its
energy diagram would look like the one for cellulose above? Of course not. It
must have a very low activation energy, Ea. That leads to an extremely
fast formation of hot gaseous products, an explosion (despite the relatively
smaller difference in energy between "nitro" and the products).
Explosives form hot gases so rapidly because they all have oxygen atoms as part
of their molecules. Thus, those molecules don't need to wait until some molecules
of atmospheric oxygen happen to hit them -- the way most substances have to
do. Alfred Nobel was driven to invent a safer explosive when four workers and
his brother were killed in the family nitroglycerin plant. He made what he called
"dynamite" when he mixed oily nitroglycerin with some powdery silica
material to form a seemingly dry solid that could be pressed into stick shape.
They didn't detonate just from being hit or dropped. Obviously, therefore, an
energy diagram for dynamite must look like the dotted line, a considerably higher
Ea indicating that more energy must be put in, e.g., by a blasting cap, to initiate
the spontaneous decomposition of the nitroglycerin. (TNT, used in armor piercing
shells, is about six times more resistant to shock than nitroglycerin. Thus,
you can guess at TNT's activation energy for reaction.) Dynamite has been
mainly replaced by other explosives for excavation, etc., today.
There. We've seen some substances with low activation energies
but we don't often handle nitro or TNT! How about a more important problem to
many of us, rusted iron, the result of iron reacting with oxygen to form iron
oxide. Of course, I'm running the risk here of opening the whole can of worms
about human activity and the second law.
—> Next <— Back
Home —>> Last


 For
instance, you've heard about the dangers of nitroglycerin, a liquid that explodes
violently just from being shaken hard or jarred sharply. Do you think that its
energy diagram would look like the one for cellulose above? Of course not. It
must have a very low activation energy, Ea. That leads to an extremely
fast formation of hot gaseous products, an explosion (despite the relatively
smaller difference in energy between "nitro" and the products).
Explosives form hot gases so rapidly because they all have oxygen atoms as part
of their molecules. Thus, those molecules don't need to wait until some molecules
of atmospheric oxygen happen to hit them -- the way most substances have to
do. Alfred Nobel was driven to invent a safer explosive when four workers and
his brother were killed in the family nitroglycerin plant. He made what he called
"dynamite" when he mixed oily nitroglycerin with some powdery silica
material to form a seemingly dry solid that could be pressed into stick shape.
They didn't detonate just from being hit or dropped. Obviously, therefore, an
energy diagram for dynamite must look like the dotted line, a considerably higher
Ea indicating that more energy must be put in, e.g., by a blasting cap, to initiate
the spontaneous decomposition of the nitroglycerin. (TNT, used in armor piercing
shells, is about six times more resistant to shock than nitroglycerin. Thus,
you can guess at TNT's activation energy for reaction.) Dynamite has been
mainly replaced by other explosives for excavation, etc., today.
For
instance, you've heard about the dangers of nitroglycerin, a liquid that explodes
violently just from being shaken hard or jarred sharply. Do you think that its
energy diagram would look like the one for cellulose above? Of course not. It
must have a very low activation energy, Ea. That leads to an extremely
fast formation of hot gaseous products, an explosion (despite the relatively
smaller difference in energy between "nitro" and the products).
Explosives form hot gases so rapidly because they all have oxygen atoms as part
of their molecules. Thus, those molecules don't need to wait until some molecules
of atmospheric oxygen happen to hit them -- the way most substances have to
do. Alfred Nobel was driven to invent a safer explosive when four workers and
his brother were killed in the family nitroglycerin plant. He made what he called
"dynamite" when he mixed oily nitroglycerin with some powdery silica
material to form a seemingly dry solid that could be pressed into stick shape.
They didn't detonate just from being hit or dropped. Obviously, therefore, an
energy diagram for dynamite must look like the dotted line, a considerably higher
Ea indicating that more energy must be put in, e.g., by a blasting cap, to initiate
the spontaneous decomposition of the nitroglycerin. (TNT, used in armor piercing
shells, is about six times more resistant to shock than nitroglycerin. Thus,
you can guess at TNT's activation energy for reaction.) Dynamite has been
mainly replaced by other explosives for excavation, etc., today.