Shakespeare and Thermodynamics:
Dam the Second Law!
The Human Importance of Activation Energies
Frank L. Lambert, Professor Emeritus (Chemistry), Occidental College, Los Angeles.
C. P. Snow's aphorism about Shakespeare and the second law of thermodynamics is meaningless to nonscientists if they are not told simply what the second law is and what it predicts about the behavior of common things. Sir Charles never did so. Equally important to nonscientists is hearing about activation energies — the barriers or "dams" to second law predictions. This web page is for individuals in the humanities and the arts or other professions so that they can sense the remarkable importance of activation energies in understanding the working of our second-law world.
In 1959 Sir Charles Snow startled several gatherings of nonscientists by saying, in effect, that their lack of knowledge of the second law of thermodynamics was equivalent to scientists not ever reading a work of Shakespeare [1]. Of course, he was using the second law as a symbol. His primary concern was to discuss the undesirability of the lack of communication between what he saw as two cultures, the humanities and sciences. However, if a speaker holds up a symbol which means little to the listeners, that action itself may further hinder communication rather than help it.
What is the validity of juxtaposing the second law with Shakespeare?
Shakespeare and the Second Law?
Shakespeare speaks movingly and powerfully about our complex human relationships. His work "teem[s] with the most vital ideas about the inner development of man, showing the whole grandeur and misery of human existence" [2]. In contrast to Shakespeare's symphony of contributions to an understanding of being human, facts and theories about the physical world like the second law may initially sound tinny and trivial. But every microsecond of our acquaintance with grandeur or with misery involves — and often is determined by — physical and chemical events outside of our bodies and inside them. Physical matter is not a trivial component of our being human, of being complex emotional and rational creatures. Externally, we may be daily both beset and delighted by natural objects and by artifacts; our lives may be straitened to desperation by simple impacts or in violent car accidents. Internally, the biochemistry of our bodies depends upon material molecules in palpable organs and plays a significant part in everyone's inner (emotive) development — as well as being responsible for our continuing life.
The importance of the second law of thermodynamics in our lives? When second law concepts are coupled with examples of the effect of activation energies on chemical and physical events, they become a Rosetta stone for interpreting all the matter-dependent happenings in life. They are the helix codes for the occurrence of physical events. They provide answers both to querulous as well as to anguished human questions of "Why me?" which are caused by physical misfortunes striking us and our artifacts — questions as old as the race and quite inexplicable by Shakespeare in his time. His profound insight into the human condition is lasting because human nature has not changed, but his seeing the working of the physical world was blinded by the scant knowledge of it in his era. The change since 1600 has been revolutionary, not just in knowledge but in understanding what then was often-sensed to be a strangely threatening physical world.
The Second Law: Physical Examples
For a brief introduction to the second law of thermodynamics, C. P. Snow could have emphasized only the direction of energy flow as its basic theme, perhaps in this way: Energy tends to flow from being concentrated in one place to becoming diffused and spread out and therefore less concentrated [3]. A hot frying pan just removed from the stove is the prototypical illustration of thermal energy becoming less concentrated; the heat always flows from the hot pan to the cooler room. The example is mundane but the universal applicability of the concept shows its power: All types of energy tend to spread out if they are not constrained from doing so.
Air under high pressure in a tire tends to leak or blow out, not to stay confined; gasoline, with its internal chemical energy, will remain unchanged for years but explosively reacts with the oxygen in air when sparked because it can thereby spread out some of its energy; a loud sound caused by the vibration of a speaker cone moving the air near it obviously does not stay confined near the speaker; a speeding car colliding with a brick wall disperses its enormous kinetic energy by twisting metal, in moving parts of the wall, in sound, in some heat; walking erect, we humans are in a precarious position energetically — we have potential energy that is converted to kinetic energy if we fall. That kinetic energy tends to spread out, perhaps only causing trauma in compressing our soft tissues if we should strike a sidewalk but perhaps breaking major bones. (Obviously, with greater kinetic energy when we fall from a horse or when we are in a car accident, the result can be a fractured spine or skull leading even to paraplegia or death.) Potential, kinetic, thermal, electrical, sonic, chemical, electromagnetic energy — all tend to disperse but, as we shall see and understand, this is a universal tendency that the second law of thermodynamics speaks of, not always an immediate process.
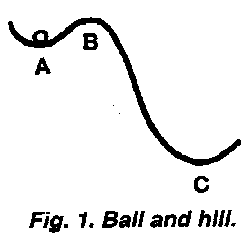 An example of the tendency of gravitational potential energy to dissipate is a system consisting of an actual soccer ball and a miniature hill. The location of the ball in a little hilltop valley, as at A in Figure 1 in contrast to position C, well illustrates the concepts of metastable versus stable energetic states. The ball has gravitational potential energy concentrated in it because it was raised from the gravitationally most stable position in this system, C, to a higher level, A. At A the ball is energetically metastable because the small hill between A and C prevents it from following the second law by dissipating its energy in rolling down to stability at C. (At any position between A and C, the ball would be in an unstable state, with positions from B to C more dramatically so.) If the ball at A is given only a modest input of energy to push it up a trace beyond the crest of the little hill, the ball will follow the second law by dissipating its energy as it rolls down to stability at C (after cycling back and forth past C, dispersing energy to the air and to the rough hill surface).
An example of the tendency of gravitational potential energy to dissipate is a system consisting of an actual soccer ball and a miniature hill. The location of the ball in a little hilltop valley, as at A in Figure 1 in contrast to position C, well illustrates the concepts of metastable versus stable energetic states. The ball has gravitational potential energy concentrated in it because it was raised from the gravitationally most stable position in this system, C, to a higher level, A. At A the ball is energetically metastable because the small hill between A and C prevents it from following the second law by dissipating its energy in rolling down to stability at C. (At any position between A and C, the ball would be in an unstable state, with positions from B to C more dramatically so.) If the ball at A is given only a modest input of energy to push it up a trace beyond the crest of the little hill, the ball will follow the second law by dissipating its energy as it rolls down to stability at C (after cycling back and forth past C, dispersing energy to the air and to the rough hill surface).
Thus, so far as the potential energy due to gravity in the ball at A is concerned, the predictions of the second law are effectively "dammed" or obstructed by the small hill between A and C. From this simple illustration, the second law can be seen to be an eternal tendency rather than an instantly obeyed edict.
Chemical examples
Chemical reactions as illustrations of matter following the second law (or of being obstructed from doing so) are as readily visualized as the foregoing physical process of a ball that could roll down a hill. Everyone has seen paper or wood burn and some iron object that, over time, has become changed from shiny to rust-covered metal. These are examples of oxidation, the spontaneous reaction of these types of matter with the oxygen in air. Unlike the ball of our first example that has extrinsically been given potential energy, many materials with which we are familiar intrinsically contain greater chemical energy within their molecules than do their oxidation products. This energy relationship is diagrammed for paper (in the presence of oxygen) in Figure 2, with the products of its burning (i.e., its oxidation), carbon dioxide and water, having less energy than the reactants of paper and oxygen, When paper reacts with oxygen, that difference in internal energy between the reactants and products is spread out to the environment as considerable heat and incidental light. Such a dispersal of some of a substance's internal energy is not just characteristic of the behavior of coal, petroleum products and wood and paper with oxygen. It is the general pattern for most common chemical reactions of a substance with another.
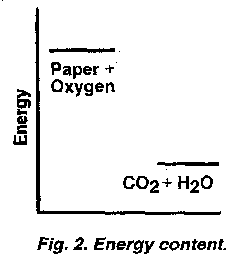 Burning and rusting can therefore be seen as a single everyday process that
exemplifies the second law, as do the myriad of less commonly known chemical reactions in our world and in the biochemistry of our bodies. Any substances that have a greater energy content than the products that would result from their interaction, as diagrammed for paper and oxygen in Figure 2, tend to change into those products because this would result in the difference in energy being diffused or spread out to the environment rather than remaining contained within the original substances. However, there is a catch. Why doesn't all paper — if it has so much energy concentrated within it — immediately catch fire in air? Fats and carbohydrates and proteins are flammable. Why don't humans and animals all spontaneously combust? What about iron? Near the ocean, it rusts very rapidly; in a dry desert, it rusts slowly. Somehow, comparable to the physical case of the ball in a hilltop valley, most chemical reactions must be obstructed and thus be prevented from following the second law instantly.
Burning and rusting can therefore be seen as a single everyday process that
exemplifies the second law, as do the myriad of less commonly known chemical reactions in our world and in the biochemistry of our bodies. Any substances that have a greater energy content than the products that would result from their interaction, as diagrammed for paper and oxygen in Figure 2, tend to change into those products because this would result in the difference in energy being diffused or spread out to the environment rather than remaining contained within the original substances. However, there is a catch. Why doesn't all paper — if it has so much energy concentrated within it — immediately catch fire in air? Fats and carbohydrates and proteins are flammable. Why don't humans and animals all spontaneously combust? What about iron? Near the ocean, it rusts very rapidly; in a dry desert, it rusts slowly. Somehow, comparable to the physical case of the ball in a hilltop valley, most chemical reactions must be obstructed and thus be prevented from following the second law instantly.
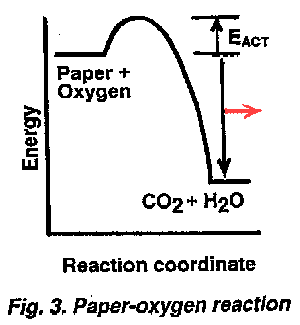 The obstruction is not a physical hill like the little A-B hill in Figure 1. Rather, it is an inherent energetic obstacle preventing reaction that must be surmounted before change to a lower energy set of products can occur and thereby allow the spreading out of the difference in energy to the surroundings [4]. As shown in Figure 3, the complete energy diagram for the reaction of paper (or forests or wooden houses) with the oxygen in air has a small energy "hill" or barrier which keeps substances from immediately following the second law. This "activation energy barrier", EACT, in Figure 3 protects oxidizable materials from immediate change by rendering them metastable rather than leaving them unstable. ("Unstable" would be represented as though the paper had just been exposed to oxygen of the air and both were teetering at the edge of the high energy plateau in Figure 2 a microsecond before they explosively reacted and formed the products in the valley below.). An amount of energy that is equal to the activation energy, EACT, (and supplied by a small amount of heat, e.g., from a match flame) must be given to some molecules of the paper and oxygen so they have enough additional energy to be at the top of the EACT barrier. Only such more energetic molecules react. However, when these first few do so, they are releasing energy as they form the lower-energy carbon dioxide and water. This dispersed energy immediately gives many nearby paper and oxygen molecules enough energy to surmount the EACT obstacle and then the process is repeated again and again until the whole paper is burned and a relatively large amount of heat (measured by the distance between the two energy levels, the downward arrow) spread out to the immediate environment (symbolized by the red horizontal arrow).
The obstruction is not a physical hill like the little A-B hill in Figure 1. Rather, it is an inherent energetic obstacle preventing reaction that must be surmounted before change to a lower energy set of products can occur and thereby allow the spreading out of the difference in energy to the surroundings [4]. As shown in Figure 3, the complete energy diagram for the reaction of paper (or forests or wooden houses) with the oxygen in air has a small energy "hill" or barrier which keeps substances from immediately following the second law. This "activation energy barrier", EACT, in Figure 3 protects oxidizable materials from immediate change by rendering them metastable rather than leaving them unstable. ("Unstable" would be represented as though the paper had just been exposed to oxygen of the air and both were teetering at the edge of the high energy plateau in Figure 2 a microsecond before they explosively reacted and formed the products in the valley below.). An amount of energy that is equal to the activation energy, EACT, (and supplied by a small amount of heat, e.g., from a match flame) must be given to some molecules of the paper and oxygen so they have enough additional energy to be at the top of the EACT barrier. Only such more energetic molecules react. However, when these first few do so, they are releasing energy as they form the lower-energy carbon dioxide and water. This dispersed energy immediately gives many nearby paper and oxygen molecules enough energy to surmount the EACT obstacle and then the process is repeated again and again until the whole paper is burned and a relatively large amount of heat (measured by the distance between the two energy levels, the downward arrow) spread out to the immediate environment (symbolized by the red horizontal arrow).
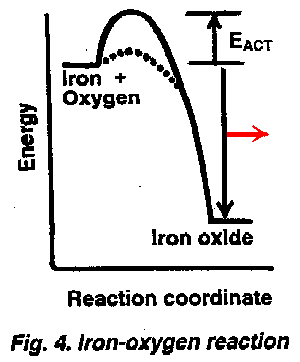
Rusting of iron is also an oxidation process but one in which the activation energies of different pathways are quantitatively different, and so, iron corrosion is a bit complex. Iron reacts with oxygen at a very slow rate in normally humid air, even though there is a sizable energy difference between the metal and its oxide, iron rust. When much moisture and some salt is present, as is true in salt-laden sea air or when iron is intermittently exposed to seawater, rusting is much faster. The difference in rate is due to differing magnitudes in the activation energies of the dry and wet processes as pictured in Figure 4. The solid line depicts the slower reaction of oxygen with iron in slightly moist air; the dotted line shows a lesser energy of activation and therefore a faster reaction of iron with oxygen and saline water (to form a slightly different oxide).
The Vital Importance of Activation Energies
All of common chemical reactions in our everyday life have sizable activation energy barriers that prevent their instantly obeying the second law. Thus, the reactants are in energetically metastable states, kept from changing in fractions of a second to form lower energy substances until a threshold amount of energy (the activation energy) is supplied to initiate a reaction. Therefore, the requirement for activation energies to start a reaction silently protects millions of different metastable substances in our artifacts, our environment, and within our bodies from undesirable change. Materials as different as ordinary natural gas and new steel or chemicals inside us from amino acids to complicated DNA can exist unchanged for long periods of time, even in our oxygen-rich environment.
The Second Law Is Time's Arrow ..
But Chemical Kinetics Is Its Clock
From the preceding simple examples, we can understand that our bodies' biochemicals and many of our prized objects, especially those made with oxidizable or other concentrated energy substances, are energetically metastable. Flammable houses, rust-prone cycles or cars or Golden Gate bridges — the direction of the flow of energy described by the second law predicts their degradation to oxides which have less concentrated-energy. But the catastrophic destruction of our flammable human artifacts does not occur without a little extra energy push from a spark or a small flame, and corrosion-caused failures don't take place in brand new metal objects. The second law of thermodynamics is correctly called "Time's Arrow" because it sharply points the direction of chemical change (from concentrated energy substances to form those with less internal energy). However, the second law is a tendency, not a firm prediction of what will happen the next moment. As we have seen, it can be delayed for long periods of time by the requirement that, for molecules to react, they must be given a small excess of their normal energy so they can surmount activation energy barriers. An extended student-level discussion is online at 2ndlaw.com/obstructions.html
The specialized area of chemistry that deals with activation energies is called chemical kinetics. Therefore, to properly summarize a valid view of the working of the physical world, Eddington's classic, "The second law of thermodynamics is time's arrow" [5] should be amended to "The second law of thermodynamics is time's arrow, but chemical kinetics is time's clock". Activation energy barriers delay and determine the time frame for the second law's predictions and thereby act as its timing mechanism. A superior metaphor expressing that temporal restriction is "Chemical kinetics firmly restrains time's arrow in the taut bow of thermodynamics for milliseconds or millennia". These are not trivial modifications of Eddington's aphorism. For more than a century popular writers, as well as philosophers and competent individuals who are not versed in chemistry, have emphasized the inevitability of the second law's predictions with little sense of the constraints, the "dams", the obstacles to its fulfillment posed by activation energies that may cause delays beyond millennia.
A fundamental statement is that of Laidler "The universe as we know it is therefore as much controlled by the laws of chemical dynamics [kinetics] as by the laws of thermodynamics" [6]. The two fields of science cannot be separated if one wants an understanding of how our real physical world works. The chemical substances in all of our artifacts as well our bodies' biochemicals are protected from instantly following the second law by inherent activation energy barriers. Thus, in a wryly anthropomorphic sense, they could be looked on as our constant protectors or even our "Maxwell's Angels" (in contrast to Maxwell's rather useless demon).
Pattern Metastability
Equally important to us is a second kind of metastability, pattern metastability [7]. A complex jet engine has very little or essentially no more total internal energy content than the original random sheets or billets of metal and other materials from which it was made. Even though an enormous amount of human labor and fossil energy was dissipated in fabricating the engine, and even though that energy and effort are reflected in the engine's price and in its human utility, there has been only slight thermodynamic energy change in it compared to the total that was in the oiriginal materials of construction. Thus, the final beautiful and powerful engine is no more threatened by the second law (in the sense of containing a larger quantity of energy and thus being more metastable energetically) than were its original components.
However, one cracked or bent turbine blade out of hundreds in this complex machine can render it almost as worthless as chunks of metal-- or far worse change it to be the cause of a plane crash. Similarly, the total DNA in a vigorous young person an instant after accidental death has no less internal energy than that same DNA a second before death; the DNA is useless because the complex molecular system ("machine") of which it was a part no longer has energy flowing through it. Metastable patterns, most strikingly those patterns involved in our metal machines or biochemical molecular machines, are the essence of what we value in most of our artifacts and what is necessary for life itself.
Yet if the patterns we call houses or cars or human beings are metastable, this means that they are always subject to becoming slightly disarrayed or, in the extreme, completely random. They are certain to be changed if an adequate amount of energy to surmount their particular "pattern barriers" strikes them. Even if they are changed only slightly by this input of energy, they will lose their singular pattern and may immediately become dysfunctional or totally nonfunctional; changed even more by energy input, they can become fragmented and random bits. However, in this wide spectrum of greater and greater loss of pattern, there has been no major loss of inherent internal energy.
The Fracture of Solid Objects and Activation Energy
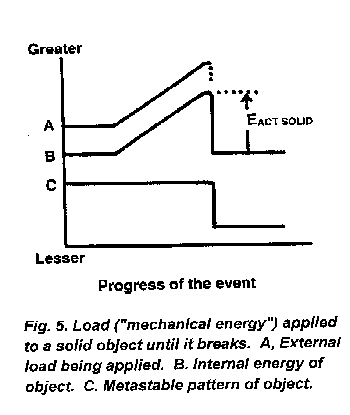 Figure 5 correlates what occurs when adequate and increasingly large load ("mechanical force") shown in line A is applied to our spinal, hip or skull bones, or to our solid artifacts whether it be a gear in any machine, a jet engine blade, a Chippendale chair, a house in mid-Florida, a freeway support in Kobe, Japan, or the Cellini salt dish in Vienna's Kunsthistorisches Museum. The middle line, B, is the internal energy of the stressed object rising gradually toward a maximum as the external load is increased. Suddenly, the fracture of the solid object occurs and line B immediately drops to its starting internal energy value (except for transient heat and the momentary kinetic energy of any flying fragments). The lower line, C, indicates the metastable pattern of the object, still functional and valuable during mild energy load, becoming immediately dysfunctional and far less valued after the fracture. Figure 5 is the diagram for a single break of a solid object. (In violent storms or earthquakes, loads are successively applied again and again to fragments and the original object may become random bits.)
Figure 5 correlates what occurs when adequate and increasingly large load ("mechanical force") shown in line A is applied to our spinal, hip or skull bones, or to our solid artifacts whether it be a gear in any machine, a jet engine blade, a Chippendale chair, a house in mid-Florida, a freeway support in Kobe, Japan, or the Cellini salt dish in Vienna's Kunsthistorisches Museum. The middle line, B, is the internal energy of the stressed object rising gradually toward a maximum as the external load is increased. Suddenly, the fracture of the solid object occurs and line B immediately drops to its starting internal energy value (except for transient heat and the momentary kinetic energy of any flying fragments). The lower line, C, indicates the metastable pattern of the object, still functional and valuable during mild energy load, becoming immediately dysfunctional and far less valued after the fracture. Figure 5 is the diagram for a single break of a solid object. (In violent storms or earthquakes, loads are successively applied again and again to fragments and the original object may become random bits.)
Our valued things (and priceless bones) are kept from the dysfunction and randomness caused by adequate external forces because of internal activation energy obstacles typified by the peak in line B or Figure 5 and identified by EACT SOLID. These kinds of barriers are due to the energy required not only to break bonds between the atoms of metal and concrete (and bone) or between the molecules of wood and plastic but also due to complex energy-requiring atomic rearrangements during the fracture [8]. Such activation energy barriers maintain the metastable patterns in our valued solid artifacts just as the conventionally depicted activation energy barriers (Figures 3 and 4) protect the continued existence of substances that are in metastable energetic states.
Metastability in Organisms
Each of us has both kinds of metastability in our bodily biochemistry: (1) about a quarter of a million different kinds of complicated, concentrated-energy (energetically metastable) molecules; and (2) thousands of different metastable patterns in which these molecules are involved — from the relationships among a myriad of quasi-static structures (cells, muscles, organs) built from individual molecules, to the intricate patterns of dynamic interacting individual molecules and groups that constitute molecular systems or "molecular machines". Molecular machines act to break food into its constituents during digestion, to transport these smaller units into the blood stream, to oxidize some substances for energy that is immediately used to keep the myriad of machines functioning or is stored, to use others for the synthesis of substances needed for structure and function, and to destroy those which are no longer useful. Systems of molecules, complexly arranged and even more complexly interacting, underlie our thinking, our memory, and our feelings. The ultimate metastable pattern is the living organism as a whole, an immense array of interconnected and functioning molecular machines operating within structures that are themselves subject to change.
Finally, the great questions involving bioenergetics and the second law arise, "How can any form of life exist if it has to stay both in metastable energetic and metastable patterned states? Why don't all living things follow the second law and quickly die either because of dissipation of their concentrated energy molecules or because of disruption of some overall structural pattern or 'molecular machine' process?" First, as we have seen, it is the activation energy barriers to the second law that keep all energetically metastable molecules in organisms from rapidly dissipating their internal energy and changing to biochemically useless compounds that have less concentrated energy. Second, metastable patterns of relationships among biological structures and metastable process patterns are protected by a vast variety of feedback systems. Biochemical sensors detect a problem in a site or a process and then chemically and electrically transmit signals to stimulate a corrective molecular machine. When the problem source is restored to its previous setpoint, or changed to the next status in its life cycle, the sensor reduces or stops transmission to the corrective biochemical machine.
Living creatures are essentially energy processing systems that cannot continue to function unless a multitude of molecular machines operate synchronically. Biochemical feedback systems counter threats to this synchroneity. Whether one's bodily patterns are threatened by excessive energy input — from desert heat or overindulgence in food.-- or by too little energy input from subsystem failure or from toxins or from physical pattern damage, feedback systems rally to hinder disastrous pattern change. Thus, an organism's feedback systems effectively protect its metastable patterns. Activation energy barriers perform as protectors in a totally different category of action, but they safeguard all the individual molecules containing concentrated energy that are constituents of molecular machines.
In summary, activation energy barriers to the second law and biochemical feedback mechanisms which act as pattern protective barriers are the keys to maintenance of our most prized artifacts and, indeed, of all living creatures in our oxygen- and energy-rich world.
The Second Law, Activation Energies, and Emotion
Sir Charles Snow's statements about Shakespeare and thermodynamics were the starting point for this discussion. Yet the preceding facts and notions do not seem to be an emotional match for a single line of Shakespeare. Not a match, that is, until the remarkable intellectual import of activation energy barriers and the immanent second law are somehow translated into the domain of emotion. This might seem to be a difficult transition.
But what could be more emotionally electrifying than, after millennia of human struggles to understand what has been seen as the threatening mystery of erratic nature, to learn why bad things happen to all of us? To be rapidly released from the prison of fear of an uncertain threatening world that is still present in so many societies and cultures? To be shown in a few minutes the ultimate reasons for the fragility of our prized possessions and to reflect on the normally sturdy protections of activation energies for our artifacts and such energies along with biochemical feedback patterns for our even more fragile biochemistry?
"When Bad Things Happen To Good People"
The anguish of the distinguished rabbi, Harold S. Kushner, was totally understandable when he was told that his brilliant young son would die in his early teens due to progeria, rapid aging. Kushner's struggle to cope and recover from this event was one of the bases for his writing "When Bad Things Happen To Good People" [9]. To a large number of individuals this was a remarkably helpful book. It may have had more voluntary readers in the U.S. from 1981 to 2000 than Shakespeare did.
No biochemist with any sensitivity or humane intelligence could have said the following to Rabbi Kushner early on, "Progeria is rare due to an infrequent error in a tiny segment of DNA and perhaps caused by a failure in one feedback step of DNA repair. However, a few cases are probably inevitable in a large population. Do you realize the infinity of complexity in the "normal" biochemistry in your own body, in your wife, in your daughter — with each of your multitude of biochemical processes functioning well this second as they have for hundreds of millions of seconds? Does not an occasional error in DNA seem to be a reasonable event?"
That would be far more than an outrageous affront to a sorrowing father. But how knowledgeable are we in this modern era if our view of the physical world is so upside down that we consider anything that is disadvantageous to us to be unwarranted and unfair? Physical nature is not clearly benign. Undoubtedly, the whole earth with its flora, and at least much of its fauna, should continually be sensed by every person to be totally beautiful. Nevertheless, the second law must be the constant physical ultimate of both scientists' and nonscientists' view of the physical world — just as activation energy barriers must be recognized as our essential bulwarks against the second law of thermodynamics' predictions.
A cold scientific statement and an untimely challenge to a grieving father are unforgivable. Just as unforgivable is the fact that so many of us do not continually take awe-full notice of how astounding is the existence of life in our earth-sun flow system. Even when we intellectually firmly realize the improbabilities, we fail to connect intellect with feelings. Inevitably therefore, we also fail to develop a comparably powerful emotional base that is not upset by no-longer-mysterious dysfunction or malformation or death
Why Bad Things Happen To All People
This brief excursion into chemical kinetics shows us clearly the cause of our failure to view physical disasters in our life right side up. It is the almost ceaseless success of unseen activation energy barriers in protecting our artifacts and ourselves from the predictions of the second law. This success makes us like spoiled overly-wealthy children — receiving so much of what we believe is due us from our servant, the physical world, that we become annoyed when one trivial activation energy barrier out of millions fails and become distraught if it is a crucial barrier.
Murphy's Law is a fraud so far as the behavior of physical objects and chemical systems is concerned. Statistically, Murphy hyperbolizes a small probability. Yet subconsciously we let his humorous law goad us to concentrate on things going wrong, and to blind us to seeing that all patterned artifacts and all life forms are unabashedly amazing. But they and we do exist. Not for moments but for years.
There are many tears in Shakespeare and in us that need not be
shed. The second law of thermodynamics is real in our physical world — it favors
immobile sand and the silent, occasionally violent, spreading out of energy.
Should not "Why me?" be our question each minute? How else can we
adequately reflect the requisite surprise and delight at simply being alive
and surrounded by pleasing and useful artifacts? Indeed, why do we cry out querulously
at the times of physically caused trouble — since antiquity and with the characters
of Shakespeare — "Why me?"..
At such times, the only rational response is "Why not me?".
But at those times that is emotionally totally unacceptable.
Activation energy barriers and our biochemical feedback mechanisms
make possible the frequency of wonderment and joy. Knowing about their occasional
failure makes the "Why me?" answerable.
Links
-
For students in the humanities and adults not in science, a more complete introduction to the second law of thermodynamics and entropy can be found at entropysimple.com.
-
For first-year college or university students who are about to begin thermodynamics,
entropysite.com/students_approach.html
is a brief summary of a modern approach to the subject.
- secondlaw.com is
a conversational, low-level no-math, introduction to the subject.
2ndlaw.com is somewhat more technical,
more keyed to microthermodynamics but
still informal and suitable for first-year students in science, except for a
brief section dealing with the non-conflict between the second law and evolution.
- entropysite.com is the site for instructors in chemistry, with a list of the many chemistry texts that have adopted our approach, and reprints of published articles concerning entropy, and supplemental material dealing with teaching the second law and entropy.
Acknowledgments
I am grateful to Professor R. E. Robertson (College of Engineering, Univ. of Michigan) for aid in developing Figure 5 and to Professor Istvan Hargittai (Budapest Technical University) for his invaluable editing and support. Special thanks are due Luu Tran, who "webified" my manuscript.
References and Notes
- Snow, C. P. The Two Cultures and the Scientific Revolution; Cambridge Univ. Press: New York,
1959, p 15. In this Rede Lecture, Sir Charles Snow reported having so challenged groups "[o]nce or
twice".
- Schumacher, E.F. Small is Beautiful; Harper & Row: New York, 1973, p 87.
- In this process, matter also tends to become more spread out or made random or "disorderly" if adequate energy flows through it. "Adequate" here means coupled (of the type and frequency which can interact with the matter, necessary) and large enough to disrupt the existing arrangement of the matter (sufficient). It is the energy flow that undergoes any entropy change, not the objects that may be rearranged by that flow. See secondlaw.com/six.html and entropysimple.com for a discussion of the fallacy of interpreting "disorder" as an increase in entropy. My invited editorial in The International Research Journal of Pure & Applied Chemistry (lambert2011.pdf) shows the sad error of Boltzmann in 1898 that led to "entropy is disorder".
- The actual cause of this "energetic obstacle", the activation energy for a reaction, is quite complex but it can be approximated by viewing the details of a chemical reaction between two substances (reactants) that together contain higher internal energy and form new substances (products) that contain lesser energy. In such a reaction chemical bonds between atoms in the reactant molecules must be broken. Bond-breaking requires large quantities of energy. In contrast, when the new bonds are formed in the molecules of the new products in this example, large quantities of energy are given out. A chemical reaction involves both processes — energy needed to break bonds, energy evolved from new bond formation — but they are not neatly simultaneous; bond-breaking must begin somewhat before bond forming can take place. Thus, the dominant effect that chemists detect in most reactions is a small energy requirement to initiate the reaction, the activation energy. However, because a great deal of energy is evolved as the new products are formed (if the products are gaseous and the process rapid, as is the case in the example of paper reacting with oxygen involving carbon dioxide and water, but not the far slower oxidation of iron), this evolved energy feeds back to supply far more than the trivial activation energy for other reactant molecules to begin breaking their bonds and for the reaction to proceed spontaneously.
- Eddington, A. S. The Nature of the Physical World; Macmillan: New York, 1928, p 74.
- Laidler, K. J. To Light Such A Candle; Oxford Univ. Press: New York, 1998, p 67.
- Pattern metastability (in the sense of an "orderly" arrangement of matter being easily — or even
inherently inevitably in time — changed into "disorderly") is often popularly linked to thermodynamic entropy, but this is a gross error, as noted above in [3] and discussed in the Web pages referred to. The change in pattern is, of course, impossible to quantify in the case of prized macro objects because of the factors of utility, of beauty, and of personal preference to humans. (The marketplace value is the only measure that represents some degree of agreement.)
- Despite their enormous importance to us in our lives and despite the fact that they involve
breaking inter-atomic or molecular bonds, fractures of solids have never been treated in chemistry. Why not? EACT SOLID is a useful qualitative idea but it cannot be quantitatively measured so that it applies to all kinds of different objects, or even to one kind of object in all "break situations". Breaks are object-specific as well as event-specific. Science treats only phenomena that can be generalized, not erratic nor unique events.
When a solid is broken — whether ski, glass goblet, bone, gear tooth, or building wall — the load required to break it and the path of the break at the molecular level is dependent on an extremely large number of variable factors: the way the object was made, its chemical composition, its shape, its ratio of surface area to volume, the strains and defects coincidentally within it, and even the rate of application of load at a particular point.
Obviously, EACT SOLID is limited to be a qualitative relationship involved in breakage, but it is a valuable and valid view because it IS related ultimately to chemical bond breaking within the solid even though a quantifiable direct relation is obscured by many variable factors.
- Kushner, H. S. When Bad Things Happen to Good People; Schocken:New York, 1981.
Copyright © 2012 Frank L. Lambert shakespeare2ndlaw.com
 An example of the tendency of gravitational potential energy to dissipate is a system consisting of an actual soccer ball and a miniature hill. The location of the ball in a little hilltop valley, as at A in Figure 1 in contrast to position C, well illustrates the concepts of metastable versus stable energetic states. The ball has gravitational potential energy concentrated in it because it was raised from the gravitationally most stable position in this system, C, to a higher level, A. At A the ball is energetically metastable because the small hill between A and C prevents it from following the second law by dissipating its energy in rolling down to stability at C. (At any position between A and C, the ball would be in an unstable state, with positions from B to C more dramatically so.) If the ball at A is given only a modest input of energy to push it up a trace beyond the crest of the little hill, the ball will follow the second law by dissipating its energy as it rolls down to stability at C (after cycling back and forth past C, dispersing energy to the air and to the rough hill surface).
An example of the tendency of gravitational potential energy to dissipate is a system consisting of an actual soccer ball and a miniature hill. The location of the ball in a little hilltop valley, as at A in Figure 1 in contrast to position C, well illustrates the concepts of metastable versus stable energetic states. The ball has gravitational potential energy concentrated in it because it was raised from the gravitationally most stable position in this system, C, to a higher level, A. At A the ball is energetically metastable because the small hill between A and C prevents it from following the second law by dissipating its energy in rolling down to stability at C. (At any position between A and C, the ball would be in an unstable state, with positions from B to C more dramatically so.) If the ball at A is given only a modest input of energy to push it up a trace beyond the crest of the little hill, the ball will follow the second law by dissipating its energy as it rolls down to stability at C (after cycling back and forth past C, dispersing energy to the air and to the rough hill surface).
 Burning and rusting can therefore be seen as a single everyday process that
exemplifies the second law, as do the myriad of less commonly known chemical reactions in our world and in the biochemistry of our bodies. Any substances that have a greater energy content than the products that would result from their interaction, as diagrammed for paper and oxygen in Figure 2, tend to change into those products because this would result in the difference in energy being diffused or spread out to the environment rather than remaining contained within the original substances. However, there is a catch. Why doesn't all paper — if it has so much energy concentrated within it — immediately catch fire in air? Fats and carbohydrates and proteins are flammable. Why don't humans and animals all spontaneously combust? What about iron? Near the ocean, it rusts very rapidly; in a dry desert, it rusts slowly. Somehow, comparable to the physical case of the ball in a hilltop valley, most chemical reactions must be obstructed and thus be prevented from following the second law instantly.
Burning and rusting can therefore be seen as a single everyday process that
exemplifies the second law, as do the myriad of less commonly known chemical reactions in our world and in the biochemistry of our bodies. Any substances that have a greater energy content than the products that would result from their interaction, as diagrammed for paper and oxygen in Figure 2, tend to change into those products because this would result in the difference in energy being diffused or spread out to the environment rather than remaining contained within the original substances. However, there is a catch. Why doesn't all paper — if it has so much energy concentrated within it — immediately catch fire in air? Fats and carbohydrates and proteins are flammable. Why don't humans and animals all spontaneously combust? What about iron? Near the ocean, it rusts very rapidly; in a dry desert, it rusts slowly. Somehow, comparable to the physical case of the ball in a hilltop valley, most chemical reactions must be obstructed and thus be prevented from following the second law instantly.
 The obstruction is not a physical hill like the little A-B hill in Figure 1. Rather, it is an inherent energetic obstacle preventing reaction that must be surmounted before change to a lower energy set of products can occur and thereby allow the spreading out of the difference in energy to the surroundings [
The obstruction is not a physical hill like the little A-B hill in Figure 1. Rather, it is an inherent energetic obstacle preventing reaction that must be surmounted before change to a lower energy set of products can occur and thereby allow the spreading out of the difference in energy to the surroundings [
 Figure 5 correlates what occurs when adequate and increasingly large load ("mechanical force") shown in line A is applied to our spinal, hip or skull bones, or to our solid artifacts whether it be a gear in any machine, a jet engine blade, a Chippendale chair, a house in mid-Florida, a freeway support in Kobe, Japan, or the Cellini salt dish in Vienna's Kunsthistorisches Museum. The middle line, B, is the internal energy of the stressed object rising gradually toward a maximum as the external load is increased. Suddenly, the fracture of the solid object occurs and line B immediately drops to its starting internal energy value (except for transient heat and the momentary kinetic energy of any flying fragments). The lower line, C, indicates the metastable pattern of the object, still functional and valuable during mild energy load, becoming immediately dysfunctional and far less valued after the fracture. Figure 5 is the diagram for a single break of a solid object. (In violent storms or earthquakes, loads are successively applied again and again to fragments and the original object may become random bits.)
Figure 5 correlates what occurs when adequate and increasingly large load ("mechanical force") shown in line A is applied to our spinal, hip or skull bones, or to our solid artifacts whether it be a gear in any machine, a jet engine blade, a Chippendale chair, a house in mid-Florida, a freeway support in Kobe, Japan, or the Cellini salt dish in Vienna's Kunsthistorisches Museum. The middle line, B, is the internal energy of the stressed object rising gradually toward a maximum as the external load is increased. Suddenly, the fracture of the solid object occurs and line B immediately drops to its starting internal energy value (except for transient heat and the momentary kinetic energy of any flying fragments). The lower line, C, indicates the metastable pattern of the object, still functional and valuable during mild energy load, becoming immediately dysfunctional and far less valued after the fracture. Figure 5 is the diagram for a single break of a solid object. (In violent storms or earthquakes, loads are successively applied again and again to fragments and the original object may become random bits.)